Onychodystrophy Testing
Various nail disorders appear identical, which makes physical examination alone less reliable. Often, nail dystrophy can have more than one cause.
Causative infectious organisms are colorless and invisible to the eye. Diagnostic testing helps you determine the exact underlying causes of nail disease.
Histopathology
Microscopic evaluation of a specimen to determine infectious or non-infectious pathologies including traumatic, inflammatory or neoplastic processes.
Periodic Acid–Schiff (PAS): Identifies non-degenerated fungal elements
Grocott Methenamine Silver (GMS): Identifies degenerated fungal elements
Fontana-Masson (FM): Identifies pigmented saprophytes and melanin pigment which may be associated with a melanocytic neoplasm
PCR
Molecular analysis of a specimen to determine if DNA of an infectious organism is present.
Onychodystrophy PCR Test: Identifies genus and species of most common fungi (dermatophytes, saprophytes, and yeasts) as well as the bacteria, pseudomonas aeruginosa, that cause nail dystrophy
PCR with Terbinafine Resistance Reflex: When positive for T. rubrum or T. mentagrophytes, detects mutations associated with terbinafine resistance
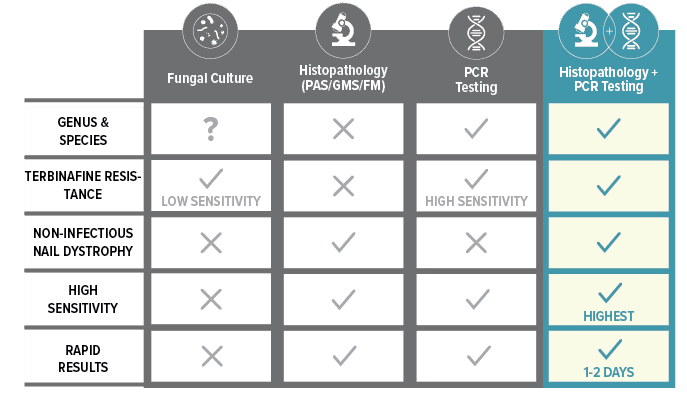
The Complete Picture
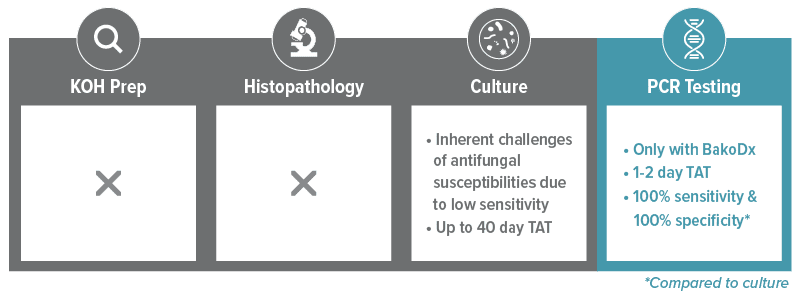
*When used in combination; Bako Nail PCR Testing: 99.9% Analytical Specificity, 86% Clinical Sensitivity. Bako’s PCR testing highly correlates with histopathology. Histopathology: 93% Clinical Sensitivity, 96% Clinical Specificity. Fungal culture: 42% False Negative Rates
Advantages of the BakoDx Onychodystrophy PCR Test
Exact
Determine the genus and species of the infectious organism to provide the safest, most effective oral and topical treatments
Accurate
Assess patient results with high confidence
99.9% analytical specificity 86% clinical sensitivity;¹ Correlates highly with histopathology
Cost-effective
Eliminate unnecessary additional testing, focus on the relevant agents of disease, and prevent treatment course changes
Required
Document the genus and species information needed for pre-authorization of many anti-fungal prescriptions
Covered
Contracted with all major insurance plans, and over 200 local insurance plans; Covered by Medicare
Rapid
Begin appropriate treatment quickly with 1-2 day turnaround time
Onychodystrophy Specimen Collection Procedure
Optimal Nail Specimen Collection for Onychodystrophy Testing
Nail Matrix Biopsy – Specimen Collection Techniques
New insights, only from BakoDx
Onychodystrophy PCR Test with Terbinafine Resistance
Know before you treat
While terbinafine is most often prescribed for onychomycosis caused by dermatophytic fungi, an increasing rate of cases with terbinafine resistance may be impeding treatment success.
To date, 12 specific genetic mutations have been identified in Trichophyton genera, primarily T. rubrum and T. mentagrophytes complex, which confer terbinafine resistance.
The new BakoDx Onychodystrophy PCR test with terbinafine resistance enables you to understand the likelihood of treatment effectiveness in days, instead of months.
How does BakoDx lab testing impact therapy outcomes, especially if a patient’s fungus is terbinafine-resistant?
Drag the slider right to see the Bako difference:
(1) Sigurgeirsson B, Olafsson JH, Steinsson JB, Paul C, Billstein S, Evans EG. Long-term Effectiveness of Treatment With Terbinafine vs Itraconazole in Onychomycosis: a 5-year blinded prospective follow-up study. Arch Dermatology 2002; 138:353–357.