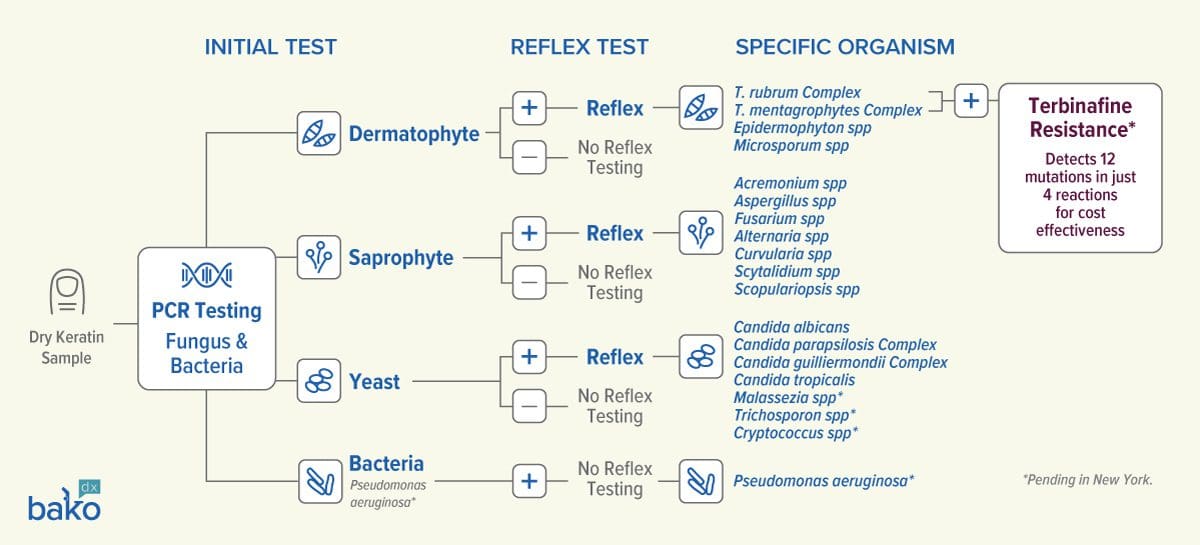
Onychodystrophy PCR Testing
Targeted Treatment Starts by Identifying the Exact Infectious Organism
-
Causative infectious organisms are colorless and invisible to the eye
-
Laboratory testing provides objective information for precise treatment
-
Determining the exact infectious agents to treat leads to improved patient outcomes and could save patients time & Rx costs
Complete the form below to get answers to your questions about BakoDx’s Onychodystrophy PCR testing with terbinafine resistance.
Please note: individual reflex test options of certain yeast, pseudomonas aeruginosa and terbinafine resistance mutations are pending availability in NY.
Advantages of the BakoDx Onychodystrophy PCR Test
with Terbinafine Resistance
Exact
Detect genus and species for targeted oral and topical antifungal therapy. Quickly determine if terbinafine treatment is likely to be effective.
Accurate
Assess patient results with high confidence.
99.9% analytical specificity 86% clinical sensitivity;¹ Correlates highly with histopathology.
Cost-effective
Save patients time and Rx costs by knowing before you treat. Focus on testing only for relevant agents of disease.
Required
Document the genus and species information needed for pre-authorization of many antifungal prescriptions.
Covered
Contracted with all major insurance plans, and over 200 local insurance plans; Covered by Medicare.
Rapid
Begin appropriate treatment quickly with 1-2 day turnaround time.
Educational Resources for Onychodystrophy
¹BakoDx internal validation
Disclaimer: BakoDx, as a partner to your practice, provides learning resources to help you better serve your patients. The information contained within this document should be used at a physician’s discretion. BakoDx assumes no liability for its content..