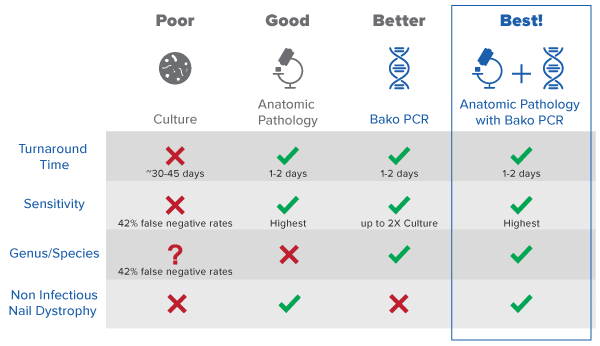
PCR
PCR-based testing identifying genetic materials for onychomycosis
PCR Turnaround Time: 24-48 Hours
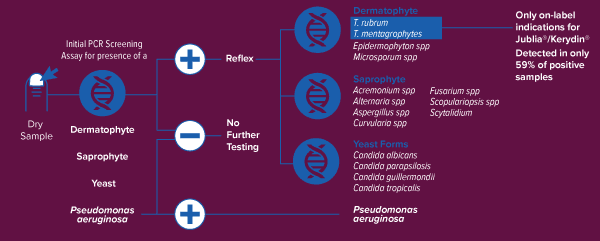
PCR Technology Workflow for Onychomycosis Testing
Bako’s proprietary, patent-pending PCR technology identifies the genus and species of the fungus causing onychomycosis enabling proper use of therapeutics and appropriate coverage within the FDA indication
click image to enlarge