ENFD Specimen Collection Procedure
The BakoDx Difference
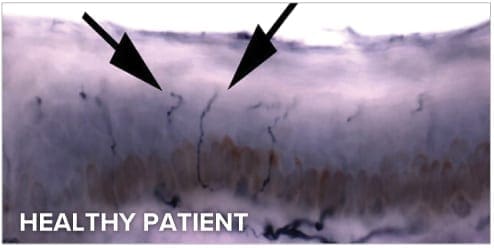
BakoDx’s ENFD reporting methodology provides an assessment of small fiber peripheral neuropathy’s degree of severity. The ENFD test report also offers treatment recommendations that empower the treating clinician with evidence-based medical information. This leads to better, more timely and cost effective health care for your patients.
Webinar: Peripheral Neuropathy Diagnosing and Treating
Case studies and procedure techniques presented by Lilly Khavari, DPM,DABPM.
We are the most experienced lab to provide Epidermal Nerve Fiber Density (ENFD) testing
Physician’s Review: BakoDx ENFD Testing
Get Your ENFD Kit
ENFD Kit(s) is complimentary for ordering clinicians
- Your account manager will contact you about scheduling an in-service and shipping your kits
- It is best to order your ENFD kit 1-2 weeks prior to the procedure date
- When you receive kit: Place cool-pack in freezer to be ready for return shipping