This is a technique for the evaluation of small fiber peripheral neuropathy
Introduction
Epidermal nerve fiber density (ENFD) testing is not a new technique; however, it is new to the diagnostic armamentarium of many in the podiatric community. This analysis is quickly becoming recognized as the gold standard among clinicians when assessing for the presence, and the degree, of small fiber peripheral neuropathy.
Though podiatric clinicians have only recently discovered this test, it has been an important diagnostic procedure for neurologists for many years. In fact, ENFD analysis has been widely used by neurologists in the United States and in Europe, to qualify and quantify small fiber peripheral neuropathy since the 1990’s.
In the following article we will discuss the meaning of “epidermal nerves” and small fiberperipheral neuropathy. We will further elaborate upon the definition of ENFD analysis, its evolution as a testing modality, its advantages over other tests, and technical aspects regarding the technique itself. Emphasis will be placed upon the feafeatures that make this test unique, and its potential uses in podiatric medicine.
What are “Epidermal Nerve Fibers”?
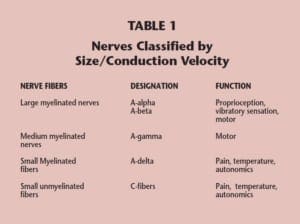
Peripheral nerves may be classified according to their function, their size, or their conduction velocity. In most instances, nerve size is directly related to conduction velocity. Nerves that have been classified based on their diameters and conduction velocities have been given specific designations. The largest myelinated peripheral nerves have been designated as A-alpha and A-beta nerves, while mediumsized myelinated nerves are, by convention, labeled A-gamma nerves. The most terminal end branches of sensory nerves represent the smallest peripheral nerves. These nerves are exceedingly small, being composed of only a few axons bundled together. By convention, these “small fibers” have been designated “A-delta” and “C” fibers, and when they terminate in the epidermis, they are called epidermal nerves (Table 1).
ENFD analysis has been widely used by neurologists in the United States and in Europe, to qualify and quantify small fiber peripheral neuropathy since the 1990’s.
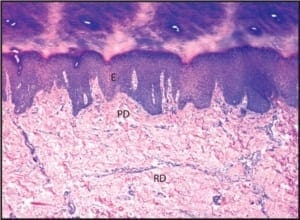
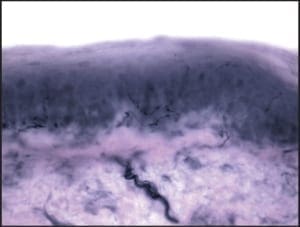
The majority of epidermal nerves are unmyelinated C-fibers. There is a much smaller subset of epidermal nerves, which possess a myelin sheath. This minor population of epidermal nerves is referred to as the “A-delta fibers”. As nerves course more superficially from the reticular dermis to the papillary dermis, and then on to the epidermis, they become progressively smaller (Figure 1).
Figure 1: A photomicrograph of normal skin, demonstrating its constituent layers. The reticular dermis has been marked with the letters RD, the papillary dermis has been annotated with PD, and the epidermis has been annotated with E. (50X)
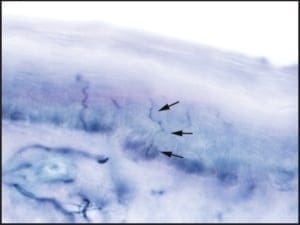
The C and A-delta fibers branch off of small nerve fascicles that course within the papillary dermis. After emerging from larger branches within the papillary dermis, these small nerve fibers run toward the skin surface, eventually entering into the viable epidermis (Figure 2).
As aforementioned, once small fibers enter into the surface epithelium, they are designated as “epidermal nerve fibers”, or more appropriately , “intra-epidermal nerve fibers.”
Figure 2: A photomicrograph demonstrating a normal epidermal nerve fiber density test. A-delta and C-fibers are seen coursing from larger branches of the papillary dermis, into the overlying epidermis. (400X)
The number of intra-epidermal nerves per a unit area of skin is termed the “epidermal nerve density”.
Paul Langerhans postulated the presence of intra-epidermal nerves a century ago; however, their presence could not be substantiated until the advent of electron microscopy in the early 1970’s. Though electron microscopy showed that intra-epidermal nerves genuinely existed, because ultra-structural examination is most effective when analyzing tissue fragments measuring only 1mm in diameter and only a few microns in thickness, this science is of limited value when trying to calculate an average density across a relatively large area of skin. The calculation of intra-epidermal nerve fiber density over a large area became possible 20 years later with the emergence of immune-histochemistry. Investigators at Johns Hopkins Medical Center performed much of the research that led to the perfection of this technique. As alluded to above, intra-epidermal nerve fibers cannot be readily visualized in routine stainedsections; rather, due to their extremely small size, they require special staining techniques. A single Epidermal nerve fiber consists of a mere 1-3 axons that are bound together with scant myelin. They have been shown to play a role in nociception (pain), temperature perception, and autonomic regulation. As they course through the epidermis, intra-epidermal fibers are thought to interact with poorly understood intra-epidermal neuro-endocrine cells known as “Merkel cells”. Roughly 90% of all intra-epidermal nerve fibers are unmyelinated Cfibers, and the remaining 10% are myelinated A-delta fibers.
In normal subjects, intra-epidermal nerves course between the squamous epithelial cells of the epidermis toward the skin surface. This intercellular path gives them a less than linear ppearance as they pass superficially (Figure 3).
Figure 3: A photomicrograph demonstrating the course of an epidermal nerve coursing between epidermal squamous cells. (1000X)
The number of intra-epidermal nerves per a unit area of skin is termed the “epidermal nerve density.” This density is relatively constant throughout life, and between genders; however, it varies widely depending on the anatomic site being tested. In general, the skin’s average fiber density decreases as one moves further from the dorsal root ganglia. In other words, the average intra-epidermal nerve fiber density is higher at the trunk than it is at the thigh, and the density in the skin of the thigh will be higher than in the skin of the distal leg.
Because the normative range of epidermal fiber density varies depending on the anatomic location, to accurately assess the meaning of the epidermal nerve fiber density at any particular site, there must be an established/published normative value for that distinct site. This is one reason that the distal leg (10cm proximal to the lateral malleolus) and proximal thigh (10cm distal to the greater trochanter of the femur) are the most commonly sampled sites. There is a massive amount of data defining the normative ranges in these locations.
What Is Small Fiber Peripheral Neuropathy?
For research purposes, intra-epidermal nerves may be locally eliminated, or “knocked out”, using exposure to low-energy shock waves or topical capsaicin. A more widespread loss of intra-epidermal nerves, leading to bonafide small fiber neuropathy, may be iatrogenic-induced through treatment with some chemotherapeutic agents. Small fiber neuropathy may arise as an occupational hazard secondary to solvent exposure or persistent vibratory forces. The latter of these commonly affects the hands of jackhammer operators and the feet of those who spend extended periods of time operating heavy equipment. Chronic alcohol abuse may precipitate small fiber peripheral neuropathy, as might systemic amyloidosis and some forms of vasculitis. Finally, patients infected with HIV commonly develop small fiber peripheral neuropathy, sometimes quite early in the onset of AIDS (Table 2).
The term “peripheral neuropathy” often has been long used as a waste-basket diagnosis into which all forms of symptomatic peripheral neuropathy have been thrown. Peripheral neuropathy is actually not a single condition, but rather, it is a descriptive term connoting any disease state, which at least in part. compromises the function of the peripheral nervous system. Peripheral neuropathy may manifest in various patterns, and may arise as the result of a wide spectrum of predisposing conditions. Small fiber peripheral neuropathy represents a large and distinct subset of the cases of peripheral neuropathy. Patients with pure small fiber peripheral neuropathy exhibit demonstrable pathology that is limited to the “small fibers” (A-delta and C fibers). This form of neuropathy may be focal, but more often involves peripheral nerves in a length-dependent pattern, meaning that the earliest and most severely affected nerves are those that are furthest away from the dorsal root ganglia. This length-dependent pattern of nerve fiber involvement gives rise to its characteristic stocking and/or glove-like distribution.
Most cases of peripheral neuropathy that are seen in a podiatric practice are of the small fiber variety; however, in some instances, large fibers may be foremost affected. It is the large fibers that are affected in patients with compression neuropathy and in demyelinating neuropathies. Even some forms of diabetic neuropathy may foremost involve large nerve fibers, as in large fiber mononeuropathy or poly-neuropathy. These large fiber neuropathies may not initially exhibit an associated small fiber component. It is the function of large fibers, not small fibers, that is tested by nerve conduction studies. This explains why some patients with frank clinical evidence of peripheral neuropathy may display normal conduction studies. Such patients suffer from pure small fiber peripheral neuropathy.
Small fiber peripheral neuropathy may be caused by a wide array of medical conditions or exposures. It has been long known that the most common causes are diabetes mellitus (types I and II) and idiopathic; however, the significance of each has been somewhat in flux in recent years. Investigators have recently revealed that many patients who were formerly thought to have idiopathic small fiber neuropathy may actually suffer from metabolic syndrome, e.g. “pre-diabetic” persons with an altered glucose metabolism.
The number of intra-epidermal nerves per a unit area of skin is termed the “epidermal nerve density”.
Epidermal Nerve Fiber Density Analysis
How are epidermal nerve fibers visualized?
As aforementioned, intra-epidermal nerve fiber density analysis usually uses the science of immunehistochemistry. Immuno-histochemical studies take advantage of the fact that the cells which make up the human body have on their surfaces antigens (usually proteins) that are relatively specific for that particular cell type. To wit, endothelial cells express an antigen designated as CD31, which is somewhat specific for the cells that line vessels, and melanocytes express a relatively specific antigen called S100 protein. It just so happens that nerves possess a particular antigen on them that has been designated as PGP 9.5. This antigen provides a specific target on intraepidermal nerves that can be used for the purpose of identification and subsequent analysis. As their name implies, immune-histochemical studies take advantage of the specific binding of antibodies (“immuno”) to identify a particular antigenic target. For intra-epidermal nerve density analysis, a small amount of PGP 9.5 is introduced into the blood of a rabbit. As a physiologic response to the presence of the foreign antigen, the rabbit will form antibodies that are highly specific for PGP 9.5. These new antibodies are then retrieved from the rabbit serum for laboratory use.
For intra-epidermal nerve fiber density analysis, clinicians most commonly perform one or two 3mm punch biopsies of skin. Once in the lab, the punch biopsy is sliced into 50μm thick sections, and each is placed in its own well within a testing tray. The rabbit-derived antibodies are then applied to each of the tissue sections. The PGP 9.5-specific rabbit antibodies will identify nerve fibers that are present within the tissue sections. The final step consists of the application of goat-derived anti-rabbit antibodies and pigment. Many goatderived anti-rabbit antibodies will bind to each of the previously applied PGP 9.5-specific rabbit antibodies. As the antibodies continue to bind, they will form a dense coat of antibody and pigment around each intra-epidermal nerve fiber. The net result is that even the tiniest nerve fibers will become visible using light microscopy.
How are epidermal nerve fiber morphology and density assessed?
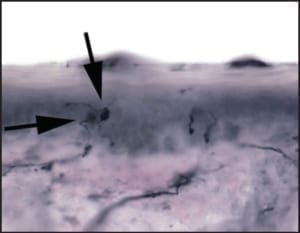
Once the fibers can be visualized, the next steps are to discern the health of the fibers by reviewing their structure and integrity, and to calculate their “density” within the epidermis. To assess nerve morphology (structure), several parameters are reviewed. Signs of degeneration include features as disparate as excessive branching, nerve thinness, nerve segmentation, poor staining, fiber varicosities, and axonal swellings (Figure 4).
Figure 4: High power magnification, demonstrating large dilatations of epidermal nerves. These axonal swellings are part of the degeneration process within diseased epidermal nerves.
When quantifying the intra-epidermal nerve fibers, first all the fibers are counted across five randomly selected 50 micron-thick tissue sections using 400X magnification. The number of intra-epidermal nerves from each representativesection is summed up. To obtain nerve density, the breadth of the epidermal surface of each crosssection is measured using image analysis software. By dividing the number of nerves by the length of the epidermal surface (in millimeters), an average density is established. The density is then expressed as the number of nerve fibers per millimeter (fibers/mm). Once the density falls below a “normal” threshold, the patient is said to have small fiber neuropathy, which may be quantified depending on the severity (Figure 5).
By far, the most studied single anatomic location on the human body is the calf at 10cm proximal to the lateral malleolus
Figure 5: High power magnification showing several attenuated and segmented epidermal nerve fibers. The total count was within the moderately neuropathic range.
It is important to note that there are several different methods of counting intra-epidermal nerves. Some labs count only those nerves that are seen crossing the epidermal basement membrane. Some investigators have found that a better counting method involves adding both those nerves that are seen crossing the basement membrane and those that are noted higher within the epidermis. This is the method used at institutions such as Johns Hopkins Medical Center and Bako Pathology Services.
Much less commonly-used techniques employ software in an attempt to create three-dimensional reproductions of the tissue to be tested. The particular manner of counting employed by a lab is of great importance because the disparate methods are not interchangeable. Each technique will elicit its own normative curve in any anatomic location. In other words, a normal count using one method might be >4 fibers/mm, but for an alternate lab (using a different counting method), the normal threshold might be >7 fibers/mm. For this reason, some consistency is warranted when monitoring a particular patient, particularly when looking for small changes secondary to oral therapy.
How does epidermal nerve fiber density analysis differ from routine pathology?
In addition to the method of staining that is used (immune-histochemistry versus routine stains), ENFD differs from routine anatomic pathology in many other ways. Whereas routine biopsies are fixed in formalin, tissue taken for ENFD analysis is not. In fact, formalin inhibits the binding of antibodies to PGP 9.5, thereby rendering the tissue useless. Biopsies taken for ENFD analysis are fixed in Zamboni’s fixative or PLP (the pros and cons of these fixatives will be discussed later). An additional difference between tissue prepared for ENFD testing, and that destined for routine pathology, is that punch biopsies being prepared for ENFD analysis are frozen and then cut into 50 micron-thick sections; in contrast, neuropatissues taken for routine pathology are dehydrated and then infused/embedded in paraffin wax so that they may be cut into 3-5 micronthick slices. Due to the greater complexity of the technique itself, from the time of receipt until the completion of slide preparation, ENFD analysis will take at least three days, whereas routine histopathology slides may be prepared in several hours.
What are the ideal biopsy sites and why?
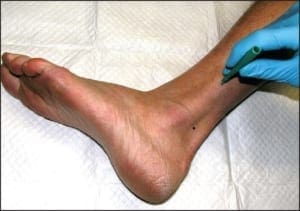
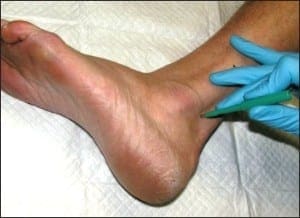
For a particular epidermal nerve fiber density quantification to have clinical significance, there must be an established normative range at the location where that sample was taken. Thankfully, potential variables such as gender and age have little influence on fiber density between individuals. The most significant single variable among normal subjects is anatomic location. This means that for a biopsy from any particular anatomic location to be useful, there must be an established normative range from that site. By far, the most studied single anatomic location on the human body is the calf at 10cm proximal to the lateral malleolus (Figure 6), followed by the proximal thigh at 10 cm distal to the greater trochanter of the femur. Normative values have been established at these locations using a variety of analytical methods.
Figure 6: The ideal anatomic location for epidermal nerve fiber density testing, 10cmabove the lateralmalleolus.
To ensure that punches are not damaged when being picked up, only atraumatic forceps should be used.
Small fiber peripheral neuropatissues thy is a length-dependent process; meaning distal anatomic sites are affected more severely than more proximal locations. For this reason, there is an advantage to taking biopsies from both the proximal thigh and distal leg, when possible. The distal biopsy is most important, offering clinicians an excellent reflection of the extent of the disease process at any point in time. It is there that the fiber density has optimal significance, and where the test is most sensitive.
In contrast, because small fiber neuropathy is length-dependent, biopsies obtained from more proximal sites may be expected to be less severely affected than distal samples in bona-fide small fiber neuropathy. In this vein, an abnormal distal biopsy and a normal proximal biopsy is evidence of length dependence, and therefore further militates in favor of small fiber neuropathy.
There is no published evidence that biopsies taken from cutaneous sites which are near to, or adjacent to, each other (such as the lower calf and dorsal foot) establish length dependence. In fact, in our experience, the second punch rarely offers enough additional information to justify the expense. Arguably, more information might be derived from performing bilateral punches. We have found that small fiber neuropathy is not always a symmetrical process, as evidenced by density values that are often not identical.
Additional sites where normal values are either being studied or pending publication are the skin posterior to the fibula, at the level of the talar dome, and over the dorsum of the foot at the level of the fourth metatarsal-cuboid articulation.
How are punch biopsies for the purpose of epidermal nerve fiber density testing obtained?
Punch biopsies taken for epidermal nerve fiber density testing are taken in a manner similar to those obtained to assess other conditions of skin with four important differences:
1) biopsies must be performed at one of a few specific anatomic locations,
2) as a standard, 3mm punch biopsies are used,
3) special care must be taken not to crush or distort the surface epithelium,
4) the biopsy is NOT fixed in formalin.
With regard to punch size, the overwhelming majority of the research on the subject has used 3mm punches. For the purposes of the test, larger punches also may be used; however, because such biopsies may require sutures and carry with them a higher complication rate, they are difficult to justify. It is primarily with differences 3 & 4 that, in our experience, clinicians may run into trouble, because in each case, if mismanaged, the very integrity of the analysis may be compromised.
Figure 5: High power magnification showing several attenuated and segmented epidermal nerve fibers. The total count was within the moderately neuropathic range.
When performing punch biopsies for the purpose of epidermal nerve fiber density analysis, it is important to avoid introducing artifact into the specimen, which might artificially alter the nerve fiber density. To avoid such artifacts, the punch blade should cut through the skin by rotating the punch back and forth between the thumb and index finger. The punch should never be forced through the skin exclusively using vertical pressure; rather, only slight downward pressure should be applied. Forcing the punch down through the skin will create a mushroom-shaped biopsy and may result in crush artifact along the biopsy’s peripheral edges. Such an artifact will likely decrease the resultant nerve fiber density.
An additional source of crush artifact is secondary to rough handling when samples are removed from the biopsy site. To ensure that punches are not damaged when being picked up, only atraumatic forceps should be used. In most instances, such forceps are included within the biopsy transport kit. The surface epidermis should never be cross-clamped when handling the biopsy. To avoid handling the surface epithelium, the sample should be gripped by the dermal soft tissue, rather than the surface epithelium. To help accomplish this, after the skin has been punched and the punch blade has been removed, the opposing limbs of the forceps may be pressed down on the skin on either side of the biopsy site. Pushing down on the surrounding skin in this manner will cause the punch itself to rise out relative to the adjacent skin. When the sample rises above the surrounding skin, the underlying dermis is exposed. It is this deeper “beefy” tissue which should be grasped with the forceps. Once the deep tissue has been grasped, the sample may be lifted out and the connective tissue base may be cut using curved scissors. An on-line demonstration of this biopsy technique may be viewed at www.bakopathology.com.
How are biopsy specimens fixated for epidermal nerve fiber density testing?
Almost all tissue that is obtained for pathologic analysis is placed in formalin; however, there are three notable exceptions, namely: tissue taken for microbiologic culture (kept fresh), tissue taken for crystal analysis due to suspected gout (placed in dehydrated alcohol), and punches taken for epidermal nerve fiber density analysis (placed in Zamboni’s fixative or PLP). Although formalin is an excellent fixative for routine anatomic pathology specimens, in tissue that is destined for epidermal nerve fiber density testing, it binds with the PGP 9.5 antigen on the surface of neurons and prevents the attachment of the anti-PGP 9.5 antibody. This lack of antibody-antigen binding will result in an erroneously diminished number of epidermal nerve fibers, even in normal subjects, and possibly lead to a falsepositive diagnosis of small fiber neuropathy.
There are two common fixatives that may be used to prepare tissue for epidermal nerve fiber analysis: PLP and Zamboni’s fixative. Each method of fixation has its advantages and disadvantages. The first fixative to be used for epidermal nerve fiber density analysis was PLP. This is an excellent fixative, but has its disadvantages. The principle disadvantages of PLP are that it has limited shelf life and must be kept refrigerated. Over the course of a few weeks following its preparation, the constituents of PLP will precipitate out, leaving the formulation ineffective for tissue fixation. This means that the kit cannot be ordered until a patient is actually scheduled for a procedure. If for any reason there is a significant delay before performing the biopsy, following the receipt of the biopsy kit, new PLP must be requested. In contrast to PLP, Zamboni’s fixative is stable at room temperature and has a much longer shelf life. When using PLP, medical practices may require a dedicated refrigerator to house their biopsy kits. This is virtually never a requirement when using Zamboni’s fixative. In addition, delays prior to performing the procedure are rarely problematic because of its long shelf life. A potential drawback to the use of Zamboni’s fixative is that it is a mild acid. Because of its corrosive properties, the biopsy specimen can only be exposed to Zamboni’s for a limited amount of time. For sufficient fixation, the specimen should be submerged in Zamboni’s fixative for at least six to eight hours; however, the analysis is best performed when the exposure time is less than 24 hours. Ideally, the biopsy should be placed in Zamboni’s fixative overnight, and then a quick rinsing step is performed early the following morning. This rinsing step removes and neutralizes the Zamboni’s fixative; it is simple to do, and takes a staff member no more than two minutes.
The full utility of epidermal nerve fiber density analysis in the management of our diabetic patient populations is only now becoming appreciated.
To perform the rinsing step, the vial containing the biopsy and Zamboni’s fixative is opened at the beginning of the day following the biopsy. The yellow Zamboni’s fixative is then poured off (leaving the skin sample in the vial). The initial vial, which should now contain only the biopsy, is then filled with phosphate buffer. The phosphate buffer is then poured off, again leaving the skin sample in its original vial. To be sure to remove or neutralize all Zamboni’s fixative, the tissue is again rinsed with phosphate buffer. Finally the vial containing the rinsed skin sample is filled with a cryoprotectant to protect the specimen during transportation. This rinsing step will ensure that the specimen is safe, even if there are short delays during shipping. Again, this biopsy rinse and transfer technique is easy to do and takes only a moment. A demonstration may be viewed online at www.bakopathology.com.
How is the biopsy for epidermal nerve fiber density handled/shipped?
Most labs that perform epidermal nerve fiber density analysis supply the materials that are essential for obtaining and shipping the sample. A basic transport kit will contain a sterile barrier, sterile punch, curved scissor, alcohol and Betadine wipes, a transport cooler and cool-pack, and prepaid overnight shipping labels. Prior to performing the biopsy procedure, the cool pack should be placed in a freezer for use during return shipping (particularly important during Jasummer months). When Zamboni’s fixative is used, the sample is fixed overnight in that fixative, then rinsed as describe above. The biopsy is then packed for shipping.
When packing the specimen, place the cool-pack into the cooler first, cover with the Styrofoam divider, and then insert the vial containing the biopsy. If Zamboni’s fixative is used, and the rinse and transfer step is deferred, clinicians must ensure that the specimen is received at the lab with 24 hours. Moreover, the sample must be received during that lab’s hours of operation so that the rinse and transfer step can be performed by laboratory personnel within the 24- hour exposure period. To minimize the chance of over-exposure, when biopsies are to be shipped in Zamboni’s fixative, they should be obtained during the afternoon hours, and then shipped to the lab on the same day for delivery the following morning.
What are the indications for epidermal nerve fiber density?
The full utility of epidermal nerve fiber density analysis in the management of our diabetic patient populations is only now becoming appreciated. Though for much of the last two decades this test has been used mostly in the context of research, it has now become a highly specific, and sensitive, method to qualify and quantify small fiber peripheral neuropathy. In fact, this test itself has played a crucial role in the research that has allowed this pattern of peripheral neuropathy to be characterized.
There are multiple angles from which epidermal nerve fiber density (ENFD) analysis will allow us to approach the management of our diabetic patients: namely, as a confirmatory diagnostic tool, as a prospective or predictive tool, and as a tool to gauge the effectiveness of medical management. The most obvious use for this examination is to definitively diagnose suspected small fiber peripheral neuropathy, particularly when a predisposing condition is not apparent.
For years, many of us in the podiatric profession have lumped all patients with burning, tingling, or numbness into a single wastebasket diagnosis, e.g., “peripheral neuropathy.” By not defining the precise pattern of neuropathy, we made it impossible to assess the effectiveness of emerging therapies on specific patient populations. This can be likened to treating every bacterial infection with penicillin and then believing the antibiotic is useless because, in some instances, it doesn’t work. By precisely characterizing the form of neuropathy affecting our patients, we can look at and judge specific therapeutic modalities in their appropriate light.
Perhaps the most interesting use of this test, and the most intriguing facet of its potential future use, involves its role as a predictive tool in patients who are at risk, or who are exceedingly early in the development of small fiber peripheral neuropathy. Because ENFD analysis may reveal degenerative changes that precede an actual drop of nerve fiber density, and in some cases a decrease in epidermal nerve fiber density may precede the symptoms of neuropathy, this test may in time become a standard means of determining which patients should be placed on preventive medication prior to their development of overt symptomatology. This approach has the potential to curb the number of patients who eventually become neuropathic.
Like most testing methods, the specificity of ENFD analysis increases when the results are nearer to the extremes of the bell curve.
Finally, ENFD analysis is widely used to monitor the effectiveness of various therapeutic modalities in diabetic (and non-diabetic) patients. In recent years, we have seen several prolific public speakers in podiatry, such as Allen Jacobs, DPM, Mackie Walker, DPM,
and Lawrence Didominico, DPM, lecture on this topic. Epidermal nerve fiber density analysis allows us to document an objective baseline prior to the initiation of therapy. We can then repeat the exam after a specific interval (6-12 months) to assess disease progression, or alternatively, disease regression. It is this manner of use that is allowing for the development and refinement of specific therapeutic options for small fiber peripheral neuropathy. It is also here that the podiatry profession can play a major, if not pivotal, role in ongoing clinical and pharmaceutical research.
What is the specificity and sensitivity of epidermal nerve fiber density analysis?
Like most testing methods, the specificity of ENFD analysis increases when the results are nearer to the extremes of the bell curve. For instance, the threshold for what should be considered a “low” ENFD, using the Bako method of counting, is 7.1 epidermal nerves fibers per millimeter. At this threshold (the 10th percentile), the specificity of ENFD is about 90%; however, at 3.8 fibers per millimeter (the 5th percentile), the specificity rises to 97%. The sensitivity of ENFD analysis is roughly 70% if no effort is made to screen out cases where there is large fiber involvement; however, if a simple tuning fork is used to assess vibratory sensation (diminished sensation is indicative of large fiber involvement), the sensitivity can be increased to roughly 90%.
What are the advantages of epidermal nerve fiber density analysis over other testing methods?
The principle advantages of ENFD over other testing methods are three-fold. Foremost, ENFD is an objective analysis, meaning it is not affected by the inherent flaws that plague subjective tests such as
Semmes-Weinstein monofilaments. Secondly, ENFD analyses exhibit high sensitivity and specificity when specifically assessing for the presence of small fiber peripheral suspectneuropathy. Many common testing methodologies, such as nerve conduction studies, measure predominantly large fiber abnormalities, and have little relation to small fiber disease. The same may also be said for sural nerve biopsy. Finally, many of the available testing methods are not readily available in an office setting; rather, they mandate that the patient report to a major academic center for testing. The biopsy used for epidermal nerve fiber density analysis may be obtained in a few minutes in an office setting.
Conclusion
In summary, epidermal nerve fiber density is a test which allows for the diagnosis of small fiber peripheral neuropathy in an objective manner, based on the analysis of a common punch of skin. The biopsy technique has subtle differences from a standard punch biopsy, some of which are crucial to the integrity of the test, namely, the biopsy size, site, handling, and fixation. This test has proven to be highly specific and sufficiently sensitive in the diagnosis of small fiber neuropathy, and an ideal method for monitoring the disease process in patients who are undergoing medical management. An additional use as a predictive modality shows great potential and will certainly be a subject of future research.