Largely unchanged for decades, treatment methods for this potentially disabling condition are finally evolving.
Introduction
Our ability to treat adverse medical conditions has long been an admixture of miraculous accomplishment and frustrating stagnation. For every disease such as osteogenic sarcoma, where recent therapeutic breakthroughs have lifted cure rates from 10-20% to over 90%, there is a condition like pulmonary adenocarcinoma, where cure rates approach their historic controls. The management of dermatologic disorders has not offered any particular exception to this rule. There recently have been profound advances in the medical management of inflammatory conditions of the skin; however, many of those advances are the first since the advent of artificial corticosteroids several decades ago. Moreover, an effective means of medically managing some of the most aggressive neoplastic processes of skin remain elusive. For instance, the prognosis attributed to melanoma and Merkle cell tumor, once they’ve spread beyond their site of origin (stage 3 or 4), remains exceedingly poor.
The management of psoriasis has remained largely unchanged for decades. With a better understanding of the pathogenesis of this potentially disabling condition, investigators have begun targeting the precise factors that lead to its progression. This new focus has shown extraordinary promise for long term medical management, particularly in severe cases. In this report we will discuss the clinical findings, diagnosis, and evolving methods of treatment for psoriasis with special attention given to those cases that present themselves in the lower extremity.
The precise inciting event in the development of psoriasis remains unclear.
Epidemiology
The overall prevalence of psoriasis approaches 2 percent internationally; however, it has been estimated to be as high as 4.6% in the United States.1 The prevalence in children is significantly lower, calculated at 0.5% in a large series of kids ages 12-16.2 The incidence in persons of color is lower than that in Caucasians, affecting roughly 0.7 percent of Africans and Asians.1 There is no particular gender predilection.1 The epidemiological features of palmoplantar (acral) psoriasis has remained somewhat obscure; however, in our experience, neither palmoplantar pustulosis nor the chronic plaque form of acral psoriasis can be considered rare in a podiatric practice, and both are certainly under-diagnosed. There is a much rarer form of pustular psoriasis which is limited to the digits and/or nails. This rare variant is designated “acrodermatitis continua”.
There are two peak ages for the development of psoriasis, the first being during early adulthood and the second being during the sixth decade of life.3 Cases manifesting in childhood are far more likely to be associated with HLA-Cw6 and a positive family history.4,5 Such patients also trend toward more severe forms of psoriasis with associated arthritis and nail involvement.6
Pathogenesis
The pathogenesis of psoriasis has been much studied and fairly well characterized; however, the precise inciting event in its initial development remains unclear.7 Psoriasis is fundamentally an autoimmune disease whereby CD4-positive, and to a lesser extent CD8-positive, T lymphocytes are stimulated to evoke a type-1 inflammatory response. Central to this response is the liberation of pro-inflammatory cytokines such as TNF-alpha which have the net result of producing local tissue damage and increasing the turnover rate of the epidermis. Where normal squamous epithelial cells require roughly 15 days to traverse from the basal layer to the stratum corneum, in affected epithelium, this rate is reduced to 1-3 days.8 This rapid turnover of affected squamous epithelium results in the silver scale that is characteristic of psoriasis. Due to the rapidity with which affected epidermis turns over, there is insufficient time for the formation of a normal stratum corneum. In some instances there may be a genetic component to the development of psoriasis. Its inheritance is in the form of a polygenic trait, resulting in the development of psoriasis in 14% of offspring when 1 parent has the disease, and 41% of offspring if both parents are affected.9 There are associations with various major histocompatibility complex phenotypes, particularly HLA B13, HLA B17, and HLA Cw6. The presence of HLA Cw6 antigens confers a relative risk of 13 for the development of psoriasis in the Caucasian population.10 A major gene involved in the development of psoriasis has recently been mapped to chromosome 6p21.3 and has been designated as PSORS1.11 Psoriasis may come and go without apparent reason; however, there are also numerous potential triggers. In many instances, psoriasis is distributed upon sites that are most affected by friction or pressure. This is the result of Koebner phenomenon, a term used to denote conditions that may be catalyzed by external trauma. Other triggers include alcohol ingestion, HIV infection, streptococcal pharyngitis (guttate variant of psoriasis), certain drugs (lithium, glucocorticoids, and beta-blockers), and emotional stress.12,13 Emotional stress plays a particularly important role as a trigger in children.12
Acral psoriasis is limited to the volar surfaces of the hands and/or feet in 81% of cases.
Clinical Findings
In the most common form of psoriasis, well-delineated geographic plaques form upon sites that are predisposed to external trauma. This common form of psoriasis has been aptly designated as the chronic plaque form of psoriasis vulgaris. When following its classic pattern of distribution, the knees, elbows, sacral region, and scalp are affected. Focal involvement of the skin is not uncommon even in the chronic plaque form of psoriasis, where approximately 2/3’s of patients develop a limited and relatively mild expression of the disease.14
Chronic plaque form psoriasis is characteristically covered by “silver” scales which when removed produced small areas of pin-point bleeding (Auspitz sign). Such silver scales are less prominent on the palms and soles. When the volar surfaces of the hands and/or feet are involved, the condition is referred to as acral psoriasis. This form of psoriasis breaks with the pattern of distribution exhibited by routine psoriasis vulgaris. Acral psoriasis is unique in that it is limited to the volar surfaces of the hands and/or feet in 81% of cases.9 It is patients with this form of psoriasis that most commonly present themselves to podiatric clinicians. There are many potential nail manifestations in psoriasis. Such pathologic changes vary from the classically described pitting and “oil spots” to onycholysis and frank keratinizing nail dystrophy. Though one set of investigators estimated the frequency of nail involvement to be roughly 80%, the true rate of involvement is probably slightly lower.15 Such overestimation is probable because bona fide keratinizing psoriatic nail unit dystrophy may be clinically indistinguishable from onychomycosis, idiopathic onycholysis, and traumatically induced dystrophy. In the lower extremity, keratinizing nail unit dystrophy (resembling onychomycosis) (Figure 1)
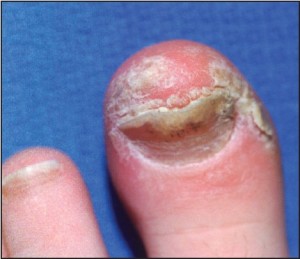 Figure 1: Psoriatic nail involvement most commonly presents as a keratinizing nail unit dystrophy (Courtesy Sean VanMarter, DPM).
Figure 1: Psoriatic nail involvement most commonly presents as a keratinizing nail unit dystrophy (Courtesy Sean VanMarter, DPM).
is much more likely to be identified than is pitting of the nail plate or “oil spots” (Figure 2).16
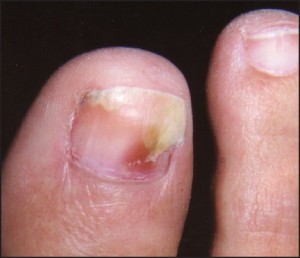 Figure 2: “Oil pits” may be seen in the lower extremity; however, they more commonly manifest on the fingers (courtesy Andrew Levy, DPM).
Figure 2: “Oil pits” may be seen in the lower extremity; however, they more commonly manifest on the fingers (courtesy Andrew Levy, DPM).
In addition to the chronic plaque-forming type of psoriasis, there are more eruptive or acute forms of psoriasis, descriptively designated as pustular and guttate (drop-like) psoriasis. Although these forms of psoriasis may precede or co-exist with chronic plaque psoriasis, due to their unique clinical presentations, they are by convention considered separately. The topic of acral psoriasis warrants separate discussion because of its tendency toward unusual clinical presentations, its recalcitrance to first line therapeutic modalities, and its common role as a diagnostic pitfall. Acral psoriasis includes those cases of psoriasis that predominantly involve the volar (non-hair-bearing) surfaces of the hands and/or feet. As aforementioned, in the overwhelming majority of cases, persons with this variant of psoriasis lack skin involvement beyond the acral surfaces. While chronic plaque form psoriasis may affect the feet in its classic form (Figure 3a and 3b),
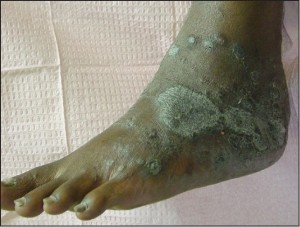 Figure 3a: Chronic plaque form psoriasis may affect the feet in its classic form, particularly on the dorsal surfaces (courtesy Brenna Steinberg, DPM).
Figure 3a: Chronic plaque form psoriasis may affect the feet in its classic form, particularly on the dorsal surfaces (courtesy Brenna Steinberg, DPM).
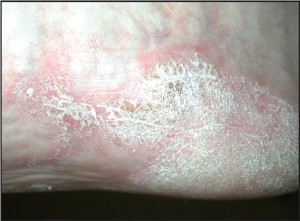 Figure 3b: Occasionally plantar lesions may also demonstrate the classic silver scales (courtesy Don Heilala, DPM).
Figure 3b: Occasionally plantar lesions may also demonstrate the classic silver scales (courtesy Don Heilala, DPM).
acral plaques are often less likely to demonstrate the characteristic silver scales that are typical of chronic plaque psoriasis when it arises in non-acral sites (Figure 4a and 4b).
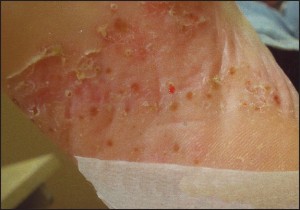 Figure 4b
Figure 4b
Figure 4a and 4b: Plaques in acral psoriasis are less likely to demonstrate silver scales characteristic of chronic plaque psoriasis (courtesy J Percival, DPM).
In some cases, acral psoriasis presents as a diffuse keratoderma with thick gray keratotic plaques (Figure 5).
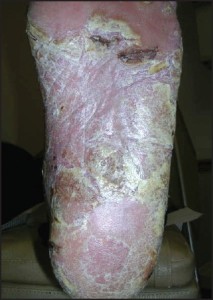 Figure 5: In some cases, acral psoriasis presents as a diffuse keratodermawith thick gray keratotic plaques (courtesy M.Gagnon,DPM).
Figure 5: In some cases, acral psoriasis presents as a diffuse keratodermawith thick gray keratotic plaques (courtesy M.Gagnon,DPM).
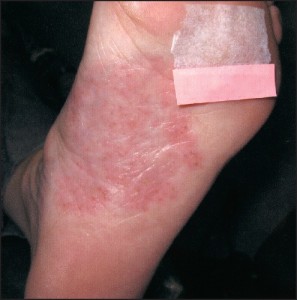
Alternatively, acral plaques may be poorly demarcated with erythematous fine scale (Figure 6),
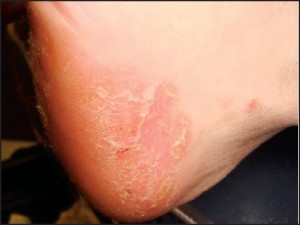 Figure 6: Acral plaques may be poorly demarcated with erythema and a fine scale (courtesy BenWeaver, DPM).
Figure 6: Acral plaques may be poorly demarcated with erythema and a fine scale (courtesy BenWeaver, DPM).
or might present as round coinshaped lesions that are reminiscent of nummular dermatitis (Figure 7).
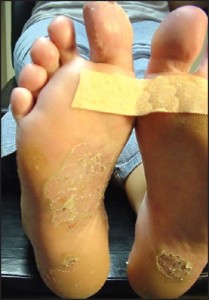 Figure 7: Acral psoriasis may present as coin-shaped lesions reminiscent of nummular (eczematous) dermatitis (courtesy Barry Blass, DPM).
Figure 7: Acral psoriasis may present as coin-shaped lesions reminiscent of nummular (eczematous) dermatitis (courtesy Barry Blass, DPM).
Regarding distribution, some cases may not only involve, but are also limited to, the forefeet (Figure 8).
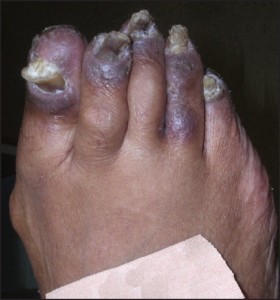 Figure 8: Some cases of acral psoriasis may be limited to the forefeet (courtesy Bruce Theall, DPM).
Figure 8: Some cases of acral psoriasis may be limited to the forefeet (courtesy Bruce Theall, DPM).
In many instances, psoriasis will consist of only one or a few discrete plaques, while in others it involves the entire plantar surface. There may, or may not, be associated nail involvement and such nail involvement may be seen in isolation (Figure 9).
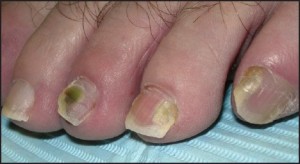 Figure 9: Psoriatic nail involvementmay be seen in isolation.
Figure 9: Psoriatic nail involvementmay be seen in isolation.
The differential diagnosis is limited when chronic plaque psoriasis vulgaris presents in its classic distribution; however, because the acral variant may lack many of the distinguishing features for psoriasis (characteristic distribution, silver scale, well-demarcated plaques, Auspitz sign), it may be confused with a wide variety of dermatological conditions. Focal presentation of the chronic plaque form of psoriasis may be confused with conditions as disparate as eczematous (nummular) dermatitis, chronic allergic contact dermatitis, lichen simplex chronicus, or squamous cell carcinoma in-situ (Bowen’s disease). Acral psoriasis may also be mistaken for chronic T. rubrum dermatophytosis, acral lichen planus, pityriasis rubra pilaris, mycosis fungoides, or chronic dyshidrotic dermatitis. Finally, the pustular form of acral pustular psoriasis is commonly misdiagnosed as tinea pedis, resulting in the institution of errant anti-fungal treatment regimens for extended periods of time.
Diagnosis
There is no diagnostic dilemma when psoriasis presents with all its classic clinical features. Well demarcated plaques with silver scale over extensor surfaces may be considered psoriasis until proven otherwise.
Other helpful clinical clues to the diagnosis of psoriasis are Auspitz sign (pinpoint bleeds upon the removal of silver scales) and Koebner phenomenon (psoriatic lesions following the path of external trauma). Unfortunately,
acral psoriasis usually lacks the classic topographic distribution that is so often attributed to chronic plaque psoriasis. In addition, acral plaques often fail to exhibit the clinical features that allow them to be easily classified as psoriatic in nature. A potentially helpful sign, particularly when dealing with acral lesions, is the presence of
individual lesions in various stages of development, e.g., the presence of acute lesions (pustules), subacute
lesions (resolving pustules), and chronic plaques in the same individual.
When the diagnosis of psoriasis is in doubt, biopsies may be indicated. The technique of choice for the diagnosis of psoriasis and virtually all other inflammatory conditions of skin is punch biopsy. On the shoe-covered skin of the foot, many clinicians prefer to perform two 2mm punches biopsies rather than 1 large punch. This technique
allows for more rapid wound healing, better sampling, and reduced risk of infection. From a histopathologic perspective, performing two small punches allows for better sampling of the lesion in question. Sampling surface keratin alone will allow clinicians to rule out dermatophytosis, and may provide histopathologic features that are sugges tive of psoriasis; however, it rarely provides for a definitive diagnosis.
Treatment
Since the dawn of modern medicine, breakthroughs in the medical management of psoriasis have been few and far between. Advancements were delayed for centuries because of widespread confusion between psoriasis vulgaris and leprosy. Psoriatic patients were often expelled from society along with persons harboring Mycobacteria leprae infections in an errant attempt to ward off disease transmission. It wasn’t until the nineteenth century that these conditions were distinguished from each other, thereby allowing the development of effective therapeutic modalities for psoriasis. The oldest legitimate treatment for psoriasis was sunlight (ultra violet light). Many subsequent attempts at therapy were developed and subsequently abandoned during the 19th, 20th and early 21st centuries. As late as 1925 many purely nonsensical treatments were advocated for the treatment of psoriasis; amongst them: intramuscular mercury or bacterial antigen injections, non-protein diets, tonsil removal, and tooth extractions! 17 Despite the frightening nature of some of these early therapeutic approaches, others such as salicylic acid, coal tar, and ultra violet light (UVB) remain in use today.
When designing a treatment regimen for an affected patient, several factors must be taken into account. The clinical extent of involvement, the patient’s perception of that involvement, the subtype of psoriasis, the impact that some therapeutic modalities will have on lifestyle, and the potential side effects that medication might have.
The lower extremities may be affected by either non-acral chronic plaque form psoriasis, or acral (palmoplantar)
psoriasis. For the clinician, the significance of subtype relates to the potential for anatomic progression, the degree to which the condition will be debilitating, and its tendency toward recalcitrance. Although acral psoriasis
(cases limited to the non-hair-bearing surfaces of the sole and digits) may remain limited with regard to its anatomic scope, this variant may be extremely disabling and recalcitrant to traditional first line therapeutic
approaches. Chronic plaque form psoriasis is often less disabling, though more widespread, and tends to be more responsive to first line therapy.
Topical Corticosteroids
As a general rule, when less than 5% of the body surface area is affected by newly diagnosed psoriasis, the first line therapy should be limited to topical modalities. The possible exception would be for cases where the condition has already proven debilitating. For decades, the first line treatment of choice for limited psoriasis has been topical corticosteroids.18
Steroids of medium to suprahighpotency (Stoughten-Cornell classification 4, 5, 6, 7) are most commonly used for limited lower extremity psoriasis, either alone, or in combination with additional topical agents. Although there is technically a potential for iatrogenic Cushings syndrome after extended courses, the expression of such is rare and typically minimal. The same is true for the development of hypothalamic-pituitaryadrenal axis suppression and significant cases are usually limited to small children due to their low relative body surface area.19 Side effects such as skin atrophy and fragility are much more common complications of topical steroid therapy. Similar to other potential complications, clinically significant cases usually require long term use; in addition, those effects are most prominent on the steroid-sensitive skin of the face and intertriginous areas.18 Newer products such as fluticasone propionate have demonstrated great promise in further reducing the risk of adverse side effects. 20 Tachyphylaxis (decreased effectiveness with continued use) is a common problem with topical high potency corticosteroids; however, its presentation may be delayed or diminished by defaulting to a pulse-dosing regimen after an initial 2-3 course of b.i.d. applications. Such pulse-dosing may be accomplished by limiting applications to 3 application times over a 24 hour period once weekly.21 An alternate method of pulse dosing involves twice daily application for two consecutive days followed by 5 days off. In addition to the emergence of improved corticosteroid formulations, new vehicles such as gels and foams are gaining broad acceptance.
Vitamin D3 Analogues
Vitamin D3 analogues were introduced into the market for the treatment of psoriasis relatively recently, but have quickly joined corticosteroids as a first line therapy for the treatment of limited psoriasis. Within affected skin, vitamin D3 analogues inhibit epidermal proliferation, promote normal stratum corneum development, and inhibit neutrophil and monocyte/ histiocyte activity.22 Possibly the best known and most studied vitamin D3 analogue is calcipotriol (Dovonex); however, other examples include calcitriol (Silkis) and tacalcitol (Curatoderem). Topical calcipotriol has been shown to be effective for the treatment of limited chronic plaque form psoriasis, including the palmoplantar variant. The efficacy of the vitamin D3 analogues is roughly comparable to that of a class 2 corticosteroid such as betamethasone dipropionate.23
Dosing for calcipotriol (50ug/g) is twice daily; however, night-time application twice weekly under occlusion has been shown to be equally effective in the treatment of palmoplantar psoriasis.24 The most significant clinical improvement is usually seen in the initial 6-8 weeks after initiating therapy.
Corticosteroid—Vitamin D
Analogue Combinations
Recently, formulations combining high potency corticosteroids and vitamin D analogues have been shown to be of clinical benefit. One such formulation combines betamethasone dipropionate and calcipotriol (Dovobet®). Such products are typically applied topically once daily and their long-term use has been shown to have a high safety
profile (see below).
Topical Retinoids
Tazarotene is a topical retinoid that was recently approved for the treatment of psoriasis. This product is available in 0.05% and 0.1% concentrations with a gel or cream base. Similar to vitamin D analogues, tazarotene lacks the side effects that are associated with long term corticosteroid therapy; however, this product has its own local side effects, in particular, irritation at the treatment site. This irritation may be managed by using combination therapy with a topical corticosteroid (see below). Due to the combination of its efficacy and its local side effects, tazarotene is usually considered a form of second line therapy in the management of limited psoriasis.
Roughly half the patients using the 0.05% or 0.1% gel describe a 50% improvement in their symptoms
after 6 weeks.25 Tazarotene gel has proven more effective than fluocinonide 0.05% cream.26
Combination Regimens Using
Topical Corticosteroids
As previously alluded to, corticosteroids are not simply effective as a form of monotherapy; they are widely used in combination with, or as an adjunct to, other topical medications. When used in combination with a keratolytic agent such as 5-10% salicylic acid or 40-50% urea, the therapeutic effects of the steroid are potentiated. In addition, corticosteroids decrease irritation that may be caused by salicylic acid. Corticosteroids also calm the local irritation that might be seen in association with retinoids and vitamin D3 analogues. In one study, improved efficacy and decreased irritation was noted when combining either tazarotene with a class IV (mometasone furoate 0.1% cream) or class II (fluocinonide 0.05% cream).27 The application of tazarotene 0.1% gel on Mondays, Wednesdays, and Fridays in alternation with clobetasol propionate ointment (class I) on Tuesdays and Thursdays, has led to the remission of psoriasis in 73% of patients with psoriasis.28 The combination of calcipotriol (vitamin D3 analogue) and betamethasone dipropionate (class I corticosteroid) has also been shown to be safe and effective.29
Ultra Violet Light and PUVA
Ultra violet light may be used in the treatment of psoriasis as either ultraviolet B phototherapy or photochemotherapy using UVA in combination with systemic or topical psoralen. Soaking affected feet in baths containing 8-methoxypsoralen followed by immediate UVA irradiation has shown to be particularly beneficial for the treatment of palmoplantar psoriasis while limiting side effects.30 The use of a topical cream containing 8- methoxypsoralen followed by UVA irradiation has proven equally effective in at least one small series.31 A recent publication noted the effectiveness of light emitted from a xenon-chloride excimer laser (308 nm) in the management of palmoplantar psoriasis.32
Systemic Therapeutic
Modalities
Many of the traditional forms of systemic therapy for severe psoriasis have remained unchanged—namely, Methotrexate and Cyclosporine remain staples in the management of patients with extensive disease. One new class of drugs that has shown notable promise for the management of psoriasis has been designated as the “biologics”. The biologic agents act at the cellular level to target specific processes that are involved in the development of a particular disease. Because their mechanisms of action are specific they tend not to have the widespread side effects that are associated with more globally acting agents such as corticosteroids. The
three principle forms of biologics are monoclonal antibodies, fusion proteins, and recombinant cytokines or growth factors.33
The generic names of the monoclonal antibodies and fusion proteins have been standardized to reflect their mechanism of action. For instance, the generic names of monoclonal antibodies have the suffix -mab, and receptor–antibody fusion proteins have the suffixcept.34 Biologic agents have one or more of 4 principle mechanisms of action. They 1) reduce the number of pathogenic T-cells (alefacept), 2) inhibit T-cell activation or migration (efalizumab), 3) modulate the immune system (Ilodecakin), or 4)
block the activity of pro-inflammatory cytokines such as tumor necrosis factor (etanercept).34
The prototypical T-cell reducing agent is alefacept, which, as its name implies, is a protein fusion product combining a T-cell binding site (Lymphocyte Function Antigen- 3) and sequences from the constant region of IgG’s heavy chain. This agent functions by binding with CD2, which is an antigen that is found on the surface of T-cell lymphocytes, particularly memory effector T-cells.35 After binding, this agent induces cell death through
apoptosis.35 Current dosing regimens call for consecutive weekly intramuscular injections of 15mg alefacept
for 12 weeks followed by 12 weeks of rest. At the conclusion of the rest period, a second course may be considered. A baseline CD4+ lymphocyte count should be performed prior to the initiation of therapy and then biweekly thereafter. In one study, 57% of patients experienced at least a 50% reduction in the Psoriasis Area Severity Index (PASI). This agent has also shown to provide a durable remission. Seventy four percent of those
patients that experience >50% reduction in PASI, maintain that such for the 12 week rest period.35
The second strategy for biologic agents used in the treatment of psoriasis involves the inhibition of Tcell
activation and migration. Efalizumab is an antibody that is directed against T-cell surface antigen CD11a (a subunit of Lymphocyte Function Antigen-1). Lymphocyte function antigen-1 (LFA-1) functions in T-cell activation and T-cell adhesion to keratinocytes and vascular endothelium.36 By interfering with the function of LFA-1, efalizumab inhibits both T-cell activation and the migration of T-cells into affected skin. The treatment regimen for efalizumab consists of the subcutaneous injection of 0.7mg/kg for the initial dose followed by 1mg/kg per week thereafter.
Higher dosage regimens and extended treatment periods are currently under investigation. Initial results suggest added benefit with successive treatment cycles and higher dosages of 2mg/kg.37 The effectiveness of efalizumab appears to be slightly shy of that achieved by alefacept with a similar tendency toward durable remissions. The third mechanism by which biologic agents may influence the development and/or progression of psoriasis is through immune system modulation. Immune system modulation may be achieved by guiding the differentiation of T-cells toward forms that play a less pathogenic role in the development of psoriasis. In general, lymphocytes may follow one of two paths of differentiation. Lymphocytes that are CD4+ may differentiate into either Th1 or
Th2 phenotype and CD8+ lymphocytes may differentiate into either Tc1 or Tc2 phenotype. Lymphocytes
may be pushed toward the Th1 and Tc1 phenotypes through the influence of cytokines Il-12 and INF-gamma or toward the Th2 and Tc2 phenotypes through the modulatory effects of IL-4, IL-6, and IL-10. Psoriasis has been shown to be driven by the cytokines that are liberated by Th1 and Tc1 phenotypes.38 Chief amongst those instigating cytokines are IFN-gamma, IL- 2, and TNF-alpha.38
Interleukin-10 is a type 2 cytokine which is under-expressed in psoriatic patients. This substance actively
opposes the type 1 cytokine response; therefore it could conceivably curtail the progression of psoriasis by reducing the liberation of cytokines IFN-gamma, IL-2, and TNFalpha. Subcutaneous injections of recombinant human IL-10 (Ilodecakin) have been shown to prolong remissions when administered in combination with other products39;
however, the effectiveness of this cytokine as a stand-alone therapeutic agent in the treatment of psoriasis has thus far been disappointing. 40 Oprelvekin is a human recombinant IL-11 that has shown initial promise when administered daily in subcutaneous injections.41 This cytokine also diminishes the type 1 cytokine response; however, it further acts by decreasing both intraepidermal T-cells and keratinocyte adhesion molecules that facilitate the action of T-cells on affected epidermis. 41 Studies are currently ongoing to further assess the usefulness of these agents.The final, and possibly most promising, class of biologics for the treatment of psoriasis includes those that directly or indirectly block the activity of type-1 inflammatory cytokines. A chief target of this class of biologics is tumor necrosis factor-alpha (TNF-alpha). Tumor necrosis factor-alpha is a type-1 cytokine that functions as a potent mediator of inflammation and keratinocyte hyperproliferation. This factor plays a pivotal role in the development and escalation of psoriasis due to its excess production and liberation by T-cell lymphocytes, keratinocytes, and mast cells.42 In theory, reducing the activity of this cytokine should impede the pathogenesis of psoriasis. Etanercept (Enbrel) is a recombinant TNF-alpha receptor which is fused with the fc portion of IgG1. This product binds with TNF-alpha on cell membranes, thereby neutralizing it. This drug is administered weekly by subcutaneous injection in 25mg or 50mg doses. Investigators have shown that higher dosages and longer therapeutic regimens provide further benefit.43 In one study nearly 50% of all patients experienced either “complete clearing” or “almost complete clearing” of their lesions after 12 weeks of biweekly 50mg injections compared to 5% in the placebo group.44 An added benefit of etanercept therapy is the durability of disease remissions in those that respond favorably. The median time until relapse (<50% PASI) was 85 days with 25% of patients not experiencing relapse for 141 days.
Infliximab (Remicade) is an additional agent that inhibits the function of TNF-alpha; however, unlike etenercept, infliximab is a monoclonal antibody. Infliximab is a composite human-mouse antibody that targets TNF-alpha directly by binding it on the surface of cells. This agent is administered intravenously at 2-4 week intervals in a concentration of 5mg/kg. As many as 80% of patients with moderate to severe psoriasis can expect to have a 75% reduction in PASI and most will experience such benefits within 4 weeks.45
As a group, the biologics are extremely new agents and thus their safety profiles remain in flux. Injection site complications are the most common adverse effects associated with etanercept and tend to diminish with ongoing therapy.46 Another potential complication is an increased risk of infection, particularly upper respiratory infections. Chronic fatigue, liver toxicity, leukocytopenia and lymphopenia are rarely reported complications.46
Summary
Psoriasis is a chronic autoimmune condition of skin which may have unconventional features when arising on the acral surfaces of the hands and/or feet. Due to its less specific appearance when arising on the skin of the feet, misdiagnoses are common, potentially resulting in inappropriate treatment. First line therapy in the treatment of limited psoriasis remains topical corticosteroids; however, there is increasing use of Vitamin D3 analogues in this context. Combination therapy using both corticosteroids and Vitamin D3 analogues or corticosteroids and retinoids have been proven affective as a second line therapy. Advances in our understanding of the athogenesis of psoriasis has allowed for the introduction of a new class of effective therapeutic agents designated as the “biologics”. These medications exert their effects by directly inhibiting the function or production of the cytokines that are responsible for the development and propagation of psoriasis. Further long-term studies are needed; however, in initial research the biologics have demonstrated tremendous promise for the treatment of moderate to severe psoriasis, including debilitating variants such as that which affects the acral surfaces.
Largely unchanged for decades, treatment methods for this potentially
disabling condition are finally evolving.