Onychodystrophy DNA Test
Target Treatment with Bako’s Onychodystrophy DNA Test
We Know Nail Infections are Difficult to Diagnose & Expensive to Treat
TARGETED TECHNOLOGY. Knowing the genus/species of the causative agent with 99.9% analytical specificity allows for targeted therapy.1
ACCURATE. Correlates highly with gold standard histology with 86% sensitivity, while providing identification of genus/species.1
TIMELY. Rapid results in 24-48 hours mean the right treatment, right away.
COST EFFECTIVE. Quick identification of genus/species supports informed treatment decisions that can more quickly lead to effective outcomes.2.
REQUIRED. Many payors require precise identification of fungal species for preauthorization of anti-fungal Rx.
COVERED. Bako’s onychodystrophy DNA test is covered by Medicare and most insurance plans.
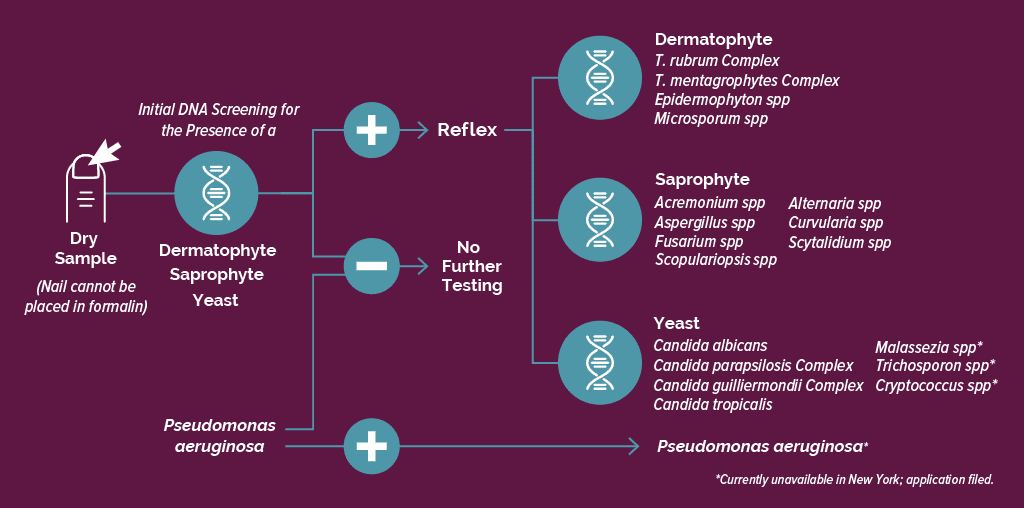
Relevant, Cost-Effective and Tiered DNA Testing Workflow
We are the only laboratory that optimizes cost using a proprietary reflex testing workflow that identifies the relevant agents of disease. Not all genus/species are treated the same.
Let Bako’s onychodystrophy DNA testing help you determine the safest and most effective oral and topical treatment.
click image to enlarge
Include Bako’s Onychodystrophy DNA Testing with Histology for the Highest Sensitivity and Specificity Diagnostic Testing Available
Gain all the advantages of Bako’s onychodystrophy DNA test, plus the added benefit of testing the sample for trauma, neoplastic processes and non-infectious nail diseases, when you include DNA testing with histopathology.
Additional Pathology Services
REFERENCES
- Bako Diagnostics’ internal data
- Based on FDA product labeling indications and usage
- Chandran, N. S., J. Y. Pan, Z. A. Pramono, H. H. Tan and C. S. Seow (2013). “Complementary role of a polymerase chain reaction test in the diagnosis of onychomycosis.” Australas J Dermatol 54(2): 105-108.
- Ghannoum, M A, et al. “A Large-Scale North American Study of Fungal Isolates from Nails: the Frequency of Onychomycosis, Fungal Distribution, and Antifungal Susceptibility Patterns.” Current Neurology and Neuroscience Reports., U.S. National Library of Medicine, Oct. 2000