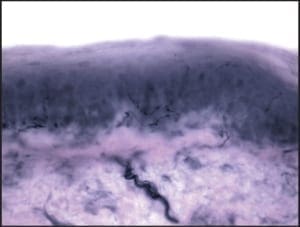
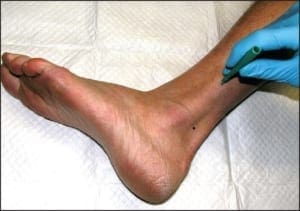
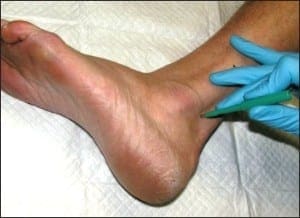
When performing punch biopsies for the purpose of epidermal nerve fiber density analysis, it is important to avoid introducing artifact into the specimen, which might artificially alter the nerve fiber density. To avoid such artifacts, the punch blade should cut through the skin by rotating the punch back and forth between the thumb and index finger. The punch should never be forced through the skin exclusively using vertical pressure; rather, only slight downward pressure should be applied. Forcing the punch down through the skin will create a mushroom-shaped biopsy and may result in crush artifact along the biopsy’s peripheral edges. Such an artifact will likely decrease the resultant nerve fiber density.
An additional source of crush artifact is secondary to rough handling when samples are removed from the biopsy site. To ensure that punches are not damaged when being picked up, only atraumatic forceps should be used. In most instances, such forceps are included within the biopsy transport kit. The surface epidermis should never be cross-clamped when handling the biopsy. To avoid handling the surface epithelium, the sample should be gripped by the dermal soft tissue, rather than the surface epithelium. To help accomplish this, after the skin has been punched and the punch blade has been removed, the opposing limbs of the forceps may be pressed down on the skin on either side of the biopsy site. Pushing down on the surrounding skin in this manner will cause the punch itself to rise out relative to the adjacent skin. When the sample rises above the surrounding skin, the underlying dermis is exposed. It is this deeper “beefy” tissue which should be grasped with the forceps. Once the deep tissue has been grasped, the sample may be lifted out and the connective tissue base may be cut using curved scissors. An on-line demonstration of this biopsy technique may be viewed at www.bakopathology.com.
How are biopsy specimens fixated for epidermal nerve fiber density testing?
Almost all tissue that is obtained for pathologic analysis is placed in formalin; however, there are three notable exceptions, namely: tissue taken for microbiologic culture (kept fresh), tissue taken for crystal analysis due to suspected gout (placed in dehydrated alcohol), and punches taken for epidermal nerve fiber density analysis (placed in Zamboni’s fixative or PLP). Although formalin is an excellent fixative for routine anatomic pathology specimens, in tissue that is destined for epidermal nerve fiber density testing, it binds with the PGP 9.5 antigen on the surface of neurons and prevents the attachment of the anti-PGP 9.5 antibody. This lack of antibody-antigen binding will result in an erroneously diminished number of epidermal nerve fibers, even in normal subjects, and possibly lead to a falsepositive diagnosis of small fiber neuropathy.
There are two common fixatives that may be used to prepare tissue for epidermal nerve fiber analysis: PLP and Zamboni’s fixative. Each method of fixation has its advantages and disadvantages. The first fixative to be used for epidermal nerve fiber density analysis was PLP. This is an excellent fixative, but has its disadvantages. The principle disadvantages of PLP are that it has limited shelf life and must be kept refrigerated. Over the course of a few weeks following its preparation, the constituents of PLP will precipitate out, leaving the formulation ineffective for tissue fixation. This means that the kit cannot be ordered until a patient is actually scheduled for a procedure. If for any reason there is a significant delay before performing the biopsy, following the receipt of the biopsy kit, new PLP must be requested. In contrast to PLP, Zamboni’s fixative is stable at room temperature and has a much longer shelf life. When using PLP, medical practices may require a dedicated refrigerator to house their biopsy kits. This is virtually never a requirement when using Zamboni’s fixative. In addition, delays prior to performing the procedure are rarely problematic because of its long shelf life. A potential drawback to the use of Zamboni’s fixative is that it is a mild acid. Because of its corrosive properties, the biopsy specimen can only be exposed to Zamboni’s for a limited amount of time. For sufficient fixation, the specimen should be submerged in Zamboni’s fixative for at least six to eight hours; however, the analysis is best performed when the exposure time is less than 24 hours. Ideally, the biopsy should be placed in Zamboni’s fixative overnight, and then a quick rinsing step is performed early the following morning. This rinsing step removes and neutralizes the Zamboni’s fixative; it is simple to do, and takes a staff member no more than two minutes.
To perform the rinsing step, the vial containing the biopsy and Zamboni’s fixative is opened at the beginning of the day following the biopsy. The yellow Zamboni’s fixative is then poured off (leaving the skin sample in the vial). The initial vial, which should now contain only the biopsy, is then filled with phosphate buffer. The phosphate buffer is then poured off, again leaving the skin sample in its original vial. To be sure to remove or neutralize all Zamboni’s fixative, the tissue is again rinsed with phosphate buffer. Finally the vial containing the rinsed skin sample is filled with a cryoprotectant to protect the specimen during transportation. This rinsing step will ensure that the specimen is safe, even if there are short delays during shipping. Again, this biopsy rinse and transfer technique is easy to do and takes only a moment. A demonstration may be viewed online at www.bakopathology.com.
How is the biopsy for epidermal nerve fiber density handled/shipped?
Most labs that perform epidermal nerve fiber density analysis supply the materials that are essential for obtaining and shipping the sample. A basic transport kit will contain a sterile barrier, sterile punch, curved scissor, alcohol and Betadine wipes, a transport cooler and cool-pack, and prepaid overnight shipping labels. Prior to performing the biopsy procedure, the cool pack should be placed in a freezer for use during return shipping (particularly important during Jasummer months). When Zamboni’s fixative is used, the sample is fixed overnight in that fixative, then rinsed as describe above. The biopsy is then packed for shipping.
When packing the specimen, place the cool-pack into the cooler first, cover with the Styrofoam divider, and then insert the vial containing the biopsy. If Zamboni’s fixative is used, and the rinse and transfer step is deferred, clinicians must ensure that the specimen is received at the lab with 24 hours. Moreover, the sample must be received during that lab’s hours of operation so that the rinse and transfer step can be performed by laboratory personnel within the 24- hour exposure period. To minimize the chance of over-exposure, when biopsies are to be shipped in Zamboni’s fixative, they should be obtained during the afternoon hours, and then shipped to the lab on the same day for delivery the following morning.
What are the indications for epidermal nerve fiber density?
The full utility of epidermal nerve fiber density analysis in the management of our diabetic patient populations is only now becoming appreciated. Though for much of the last two decades this test has been used mostly in the context of research, it has now become a highly specific, and sensitive, method to qualify and quantify small fiber peripheral neuropathy. In fact, this test itself has played a crucial role in the research that has allowed this pattern of peripheral neuropathy to be characterized.
There are multiple angles from which epidermal nerve fiber density (ENFD) analysis will allow us to approach the management of our diabetic patients: namely, as a confirmatory diagnostic tool, as a prospective or predictive tool, and as a tool to gauge the effectiveness of medical management. The most obvious use for this examination is to definitively diagnose suspected small fiber peripheral neuropathy, particularly when a predisposing condition is not apparent.
For years, many of us in the podiatric profession have lumped all patients with burning, tingling, or numbness into a single wastebasket diagnosis, e.g., “peripheral neuropathy.” By not defining the precise pattern of neuropathy, we made it impossible to assess the effectiveness of emerging therapies on specific patient populations. This can be likened to treating every bacterial infection with penicillin and then believing the antibiotic is useless because, in some instances, it doesn’t work. By precisely characterizing the form of neuropathy affecting our patients, we can look at and judge specific therapeutic modalities in their appropriate light.
Perhaps the most interesting use of this test, and the most intriguing facet of its potential future use, involves its role as a predictive tool in patients who are at risk, or who are exceedingly early in the development of small fiber peripheral neuropathy. Because ENFD analysis may reveal degenerative changes that precede an actual drop of nerve fiber density, and in some cases a decrease in epidermal nerve fiber density may precede the symptoms of neuropathy, this test may in time become a standard means of determining which patients should be placed on preventive medication prior to their development of overt symptomatology. This approach has the potential to curb the number of patients who eventually become neuropathic.
Like most testing methods, the specificity of ENFD analysis increases when the results are nearer to the extremes of the bell curve.
Finally, ENFD analysis is widely used to monitor the effectiveness of various therapeutic modalities in diabetic (and non-diabetic) patients. In recent years, we have seen several prolific public speakers in podiatry, such as Allen Jacobs, DPM, Mackie Walker, DPM,
and Lawrence Didominico, DPM, lecture on this topic. Epidermal nerve fiber density analysis allows us to document an objective baseline prior to the initiation of therapy. We can then repeat the exam after a specific interval (6-12 months) to assess disease progression, or alternatively, disease regression. It is this manner of use that is allowing for the development and refinement of specific therapeutic options for small fiber peripheral neuropathy. It is also here that the podiatry profession can play a major, if not pivotal, role in ongoing clinical and pharmaceutical research.
What is the specificity and sensitivity of epidermal nerve fiber density analysis?
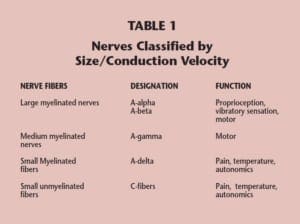
Like most testing methods, the specificity of ENFD analysis increases when the results are nearer to the extremes of the bell curve. For instance, the threshold for what should be considered a “low” ENFD, using the Bako method of counting, is 7.1 epidermal nerves fibers per millimeter. At this threshold (the 10th percentile), the specificity of ENFD is about 90%; however, at 3.8 fibers per millimeter (the 5th percentile), the specificity rises to 97%. The sensitivity of ENFD analysis is roughly 70% if no effort is made to screen out cases where there is large fiber involvement; however, if a simple tuning fork is used to assess vibratory sensation (diminished sensation is indicative of large fiber involvement), the sensitivity can be increased to roughly 90%.
What are the advantages of epidermal nerve fiber density analysis over other testing methods?
The principle advantages of ENFD over other testing methods are three-fold. Foremost, ENFD is an objective analysis, meaning it is not affected by the inherent flaws that plague subjective tests such as
Semmes-Weinstein monofilaments. Secondly, ENFD analyses exhibit high sensitivity and specificity when specifically assessing for the presence of small fiber peripheral suspectneuropathy. Many common testing methodologies, such as nerve conduction studies, measure predominantly large fiber abnormalities, and have little relation to small fiber disease. The same may also be said for sural nerve biopsy. Finally, many of the available testing methods are not readily available in an office setting; rather, they mandate that the patient report to a major academic center for testing. The biopsy used for epidermal nerve fiber density analysis may be obtained in a few minutes in an office setting.
Conclusion
In summary, epidermal nerve fiber density is a test which allows for the diagnosis of small fiber peripheral neuropathy in an objective manner, based on the analysis of a common punch of skin. The biopsy technique has subtle differences from a standard punch biopsy, some of which are crucial to the integrity of the test, namely, the biopsy size, site, handling, and fixation. This test has proven to be highly specific and sufficiently sensitive in the diagnosis of small fiber neuropathy, and an ideal method for monitoring the disease process in patients who are undergoing medical management. An additional use as a predictive modality shows great potential and will certainly be a subject of future research.