Introduction
As a general rule, the podiatic profession is notorious for its underutilization of cutaneous and soft tissue biopsy techniques. In some instances, such under-use may be attributed to trepidation stemming from a lack of familiarity with the biopsy techniques themselves; however, I believe this is not the preeminent cause. In my experience, the most common reason that many podiatric clinicians don’t use biopsy techniques to their fullest is a degree of insecurity regarding when to use witch biopsy and what to do with them once the biopsy has been performed. In most offices, the support staff is ill prepared to offer much in the way of guidance, leaving the clinicians on their own.
The purpose of this report is to summarize the indication for biopsy, when each technique is most appropriate, and the method of fixation that is required for laboratory examination.
As a general rule, the podiatric profession is notorious for its under-utilization of cutaneous and soft tissue biopsy techniques.
Biopsy Techniques
Not uncommonly, clinicians create unnecessary limitations with respect to how they should use biopsies and what constitutes a ‘biopsy’ itself. We can easily fall into a pattern of thinking whereby we equate the word ‘biopsy’ with a 3:1 ellipse or limit the word biopsy to only those specimens taken by punch technique. Either misimpression might result in under-utilization and the latter would likely result in suboptimal sampling in more than half of the cases in which a biopsy is being used. Simply stated, anytime tissue is taken to obtain a diagnosis through histopathologic analysis, a biopsy has been performed. Common techniques include, but are not limited to, punch biopsy, shave biopsy, saucerization (a form of shave biopsy), curettage, core needle, and aspiration biopsy.
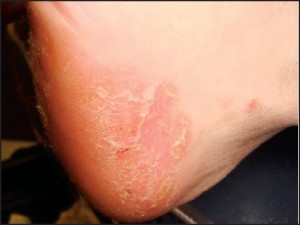
Punch biopsy (CPT 11100 initial, CPT 11101 subsequent) is a common technique used to sample conditions that 1) are too large to be shaven or 2) have a deep dermal component (requiring deeper sampling). This is the ideal technique for sampling all forms of dermatitis; however, this is far from where its utility ends. Punches may be used to sample suspected vasculitis, ulcers, large pigmented lesions (those too large to be shaven), and other large suspected neoplasms (those that are greater than 1cm in diameter). Three-millimeter punches are used for epidermal nerve fiber density testing (Figure 1).
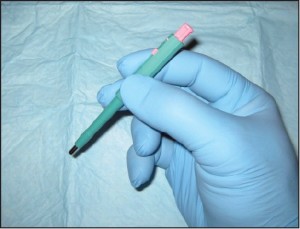 Figure 1. A 2mm punch biopsy. This technique is used for epidermal nerve fiber density testing, however a 3mm punch in necessary in that context (courtesy Carl Solomon, DPM).
Figure 1. A 2mm punch biopsy. This technique is used for epidermal nerve fiber density testing, however a 3mm punch in necessary in that context (courtesy Carl Solomon, DPM).
Small punches (2mm) also may be ideal for plantar verrucous lesions. Because this technique samples the full thickness of such plantar lesions, it allows dermatopathologists to accurately discriminate between genuine verrucae and clinically identical verrucous carcinomas. In sum, punch biopsies are ideal when a small part of a much larger lesion is submitted for histopathology.
Standard shave biopsies (CPT 11300 series) are used much more commonly in dermatologic practices than are punches. In fact, the combination of standard shaves and saucerizations) represents the overwhelming majority of biopsies performed by dermatologists. This technique uses a scalpel to “shave off” a lesion of concern (Figure 2),
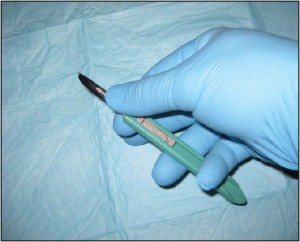 Figure 2. Standard shave biopsy. This is the procedure of choice for sampling papules and small nodules.
Figure 2. Standard shave biopsy. This is the procedure of choice for sampling papules and small nodules.
leaving a shallow iatrogenic ulceration in its wake. Shave biopsies contrast sharply with punch biopsies in many respects. Shaves do not routinely sample the deep dermis, rather, they typically extend only to the depth of the superficial dermis. In addition, where punches are relatively narrow (most punches are 2-4mm in diameter), shaves often encompass a broad sample of superficial skin (often 1cm or greater in width).
Shave technique is the ideal sampling method for unexplained papules (elevated lesions measuring 5mm or less in diameter), and can be used for macules (flat lesions measuring less than 1cm in diameter). Usually a standard shave is performed when the lesion in question appears superficial and/or exophytic (outward growing).
Clinicians create unnecessary limitations with respect to how they should use biopsies and what constitutes a ‘biopsy’ itself.
Saucerization (CPT 11300 series) is a technique that is closely related to a standard shave biopsy. In fact, saucerization has many of the same indications and is coded identically. This technique uses a bendable blade, not dissimilar to a Gillette razor blade, to “scoop out” the tissue of concern (Figure 3).
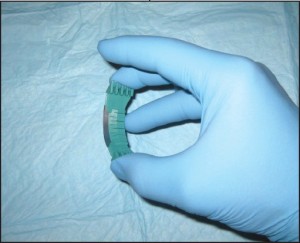 Figure 3. Saucerization biopsy. This technique is ideal when sampling flat lesions and those that require additional depth.
Figure 3. Saucerization biopsy. This technique is ideal when sampling flat lesions and those that require additional depth.
Where standard shaves are ideal for superficial and/or exophytic lesions, saucerizations are better for flat or endophytic (inward growing) lesions. Conceptually, this technique is thought of as a more aggressive method of sampling and thus can lend itself more to complete excision than can shave.
Curettage (CPT 11100 unless completely excised) is a less common sampling method than are the previously described techniques; however, it remains a viable biopsy technique for many dermatologists. Curettage uses a dermal curette to scrape the surface of the skin to obtain the desired tissue sample (Figure 4).
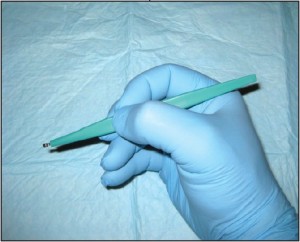 Figure 4. Curettage. This technique can be used for sampling superficial lesions which can be “scraped off” the skin surface.
Figure 4. Curettage. This technique can be used for sampling superficial lesions which can be “scraped off” the skin surface.
The disadvantage of curettage is largely related to how difficult these specimens may be to histopathologically examine. Inherent in the mechanics of this technique is the fragmentation of the sample. This may make the tissue impossible to correctly orient, which can complicate its histopathologic examination.
Core needle biopsy is an ideal method of characterizing more deeply seated masses. This technique is perfect for investigating tumors of the soft tissue. Essentially any mass that falls into the differential diagnosis with a lipoma or ganglion could be sampled using c o r e n e e d l e technique. Makers of core needles include Temno ™ and Trucut™ (Figure 5).
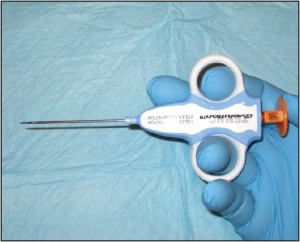 Figure 5. Core needle biopsy. Pictured is a Temno™ core needle which is ideal for harvesting tissue from subcutaneous or deep soft tissue masses.
Figure 5. Core needle biopsy. Pictured is a Temno™ core needle which is ideal for harvesting tissue from subcutaneous or deep soft tissue masses.
Core needles a r e h o l l o w , large gauge needles designed to withdraw thin columns of tissue from within the mass in question. The technique is easy to perform and is highly effective. To obtain tissue using core needle biopsy, the skin overlying the mass is lanced and the instrument is inserted to abut the mass in question. Depending on the size of the mass, the biopsy instrument is set by the clinician to sample to a desired depth using a spring loaded mechanism. The device is discharged and the sample is obtained. This is usually repeated into the masses’ various quadrants.
Needle aspiration biopsy (CPT 10021 without imaging guidance or 10022 with imaging) is slightly less invasive than is core needle biopsy but has essentially the same indications. This technique uses a large gauge hypodermic needle and a large syringe to sample fluid and/or cells from deeply seated masses (Figure 6).
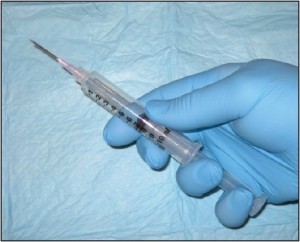 Figure 6. Needle aspiration biopsy. This is a useful technique to help guide the surgeon toward open biopsy, additional imaging, etc.
Figure 6. Needle aspiration biopsy. This is a useful technique to help guide the surgeon toward open biopsy, additional imaging, etc.
The secret of core needle biopsy is to create a vacuum once the needle has been introduced into the mass and to maintain the vacuum throughout the remaining aspiration procedure. The needle should be redirected into each of the 4 quadrants twice, without releasing the plunger and without completely removing the needle from the mass. Any fluid that is obtained may be expelled into fixative. If grossly perceivable tissue is not obtained, fixative should be drawn up into the syringe and then squirted back into the specimen container . This functions to flush any of the potentially neoplastic cells out of the aspiration needle.
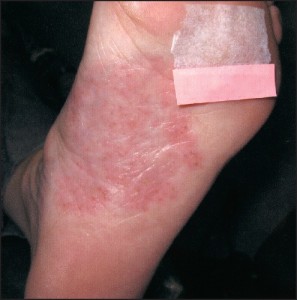
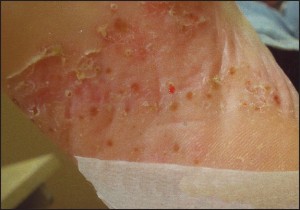
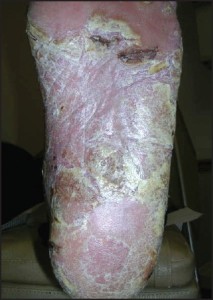
Biopsy Indications The indications for biopsy vary depending on the nature of the lesion in question. As a general rule, there are three categories of indications for biopsy: 1) atypically appearing lesions, 2) typically appearing lesions which behave in an atypical fashion, 3) progressive, longstanding lesions or conditions which cannot be adequately explained. For inflammatory conditions / dermatitis, the indications are predominantly related to the patient’s perception of the condition. Indications include, but are not limited to: 1) significant patient frustration, 2) severe symptomatology, 3) recalcitrance and 4) those cases that have entirely nonspecific clinical findings. In contrast, the indications for biopsy in relation to pigmented lesions of the skin are almost entirely related to the clinician’s impression of the lesion in question. Asymmetry of pigment, asymmetry of configuration, irregular outlines, and a diameter of greater than 6mm all represent indications for biopsy.off” the skin surface.
Pigmented lesions include all those lesions that might fall within the differential diagnosis of a pigmented malignant melanoma. This differential diagnosis includes lesions as disparate as solar lentigos, melanocytic nevi, seborrheic keratoses, tinea nigra, and talon noir. The criteria which should lead to the consideration of biopsy within this class of lesions are: 1) asymmetry of configuration, 2) asymmetry or variegation of color, 3) irregular outlines, or 4) size greater than 6mm in diameter . The symmetry of configuration should be assessed in 3 dimensions. As such, if the lesion is bisected in a plane that is perpendicular to the skin, each half should be identical, not just with regard t o i t s s h a p e along the skin’s surface, but also its elevations and depressions. It should also be stated that newly arising or changing lesions greater than 6mm in diameter in adults represent an absolute indication for biopsy, not a relative one. The indications for biopsy in relation to non-pigmented tumors of the skin are less well defined and lend themselves to more subjectivity than those for pigmented lesions. For the most part , papules, nodules, and plaques that cannot be effectively explained should receive prompt biopsy, or at minimum, clinical follow-up. Lesions that do not show evidence of resolution or involution should be sampled. An important point here is that simply harboring a papule or nodule that cannot be explained is of itself “atypical”.
Frank evidence of malignancy is not ne c e s s a ry to prompt a biopsy. In keeping with this theme, biopsies should be considered on all ulcers that cannot be adequately explained, and those that persist despite appropriately targeted therapy. Additional miscellaneous biopsy indications include: verrucous lesions in persons over 40 years of age, tumors of all types that show recalcitrance, and pyogenic granulomata in persons over 40 (Figure 7).
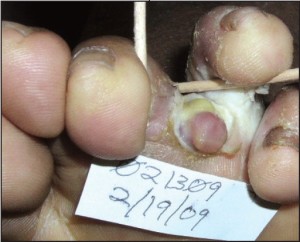 Figure 7. This Kaposi’s sarcoma in a 40 yearold male could easily be confused with a pyogenic granuloma. (courtesy Carl Solomon, DPM).
Figure 7. This Kaposi’s sarcoma in a 40 yearold male could easily be confused with a pyogenic granuloma. (courtesy Carl Solomon, DPM).
The soft tissue includes all the non-epithelial, non-skeletal, and non-visceral tissue of the body. This includes smooth muscle, skeletal muscle, adipose tissue, fibrous tissue, and peripheral neural tissue, amongst others. The indications related to biopsying tumors of the soft tissue are complicated by three facts. Foremost, tumors of the soft tissue are usually masked by the overlying skin and fascia, making their clinical features entirely nonspecific. Secondly, the foot boasts a large number of benign soft tissue masses that aren’t particularly common elsewhere within the body. Ganglia, for instance, are largely limited to the hands and feet. The same is true of digital mucous cysts. Finally, podiatric clinicians may be unaware of core needle and aspiration biopsy techniques, instead relying on more invasive open biopsies or complete excisions. This constellation of facts has the net result of diminishing the number of biopsies performed by podiatric clinicians and lowering their index of suspicion when dealing with soft tissue masses in the foot. In many instances, diagnostic procedures are delayed, resulting in tumor progression and a worsened prognosis. As a general rule, progressive soft tissue masses that cannot be adequately explained should be investigated. The dimension that is often given in the orthopedic oncology literature as an absolute indication for biopsy is 5cm; however, this is of little value when dealing with masses in the foot because of their easy accessibility. Tumors become evident at a much smaller size in the foot and thus should be investigated. Simply having a cystic component does not exclude the possibility of neoplasia as high grade sarcomas and many adnexal carcinomas can cavitate . Similarly, a mass’s tendency toward trans-illumination does not equate with benignancy .Many cystic and myxoid malignancies will trans-illuminate to some extent (Figure 8).
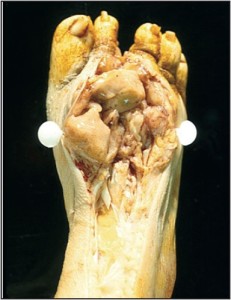 Figure 8. This high-grade fibrosarcoma was not biopsied because like a ganglion it trans illuminated.
Figure 8. This high-grade fibrosarcoma was not biopsied because like a ganglion it trans illuminated.
Imaging may be helpful; however, as many clinicians will acknowledge, this rarely provides a definitive opinion with regard to a mass’s potential biologic behavior. Early investigations may be aided by needle aspipapule ration biopsy. This technique has the advantage of being minimally invasive and well tolerated. This makes it an easy technique to perform as a purely investigative procedure, allowing clinicians to rule in or out the obvious diagnoses. Masses that fail to produce an aspirate(“dry taps”) should be taken for open biopsy, the most common form of which is core needle biopsy. When the aspiration of a mass produces fluid of an unusual consistency, it should be sent for cytopathologic analysis. Initial aspiration biopsies, which produce clear mucinous fluid typical of a ganglion, may be sent for analysis at the clinician’s discretion; however, subsequent aspirations performed on recalcitrant lesions must be analyzed.
Technique Selection
Large plaques, patches
The principles for technique selection are fairly rudimentary with few exceptions. Dermatitides and other conditions of the skin, which are too broad to “shave off”, are instead punched. Inflammatory conditions that would most often be punched include psoriasis, eczematous (nummular) dermatitis, lichen planus, granuloma annulare, and all conditions that might fall into their differential diagnosis. Neoplasms also may be punched in some instances. Squamous cell carcinoma in-situ may present as a plaque and thus warrant punch biopsy. Similarly,melanoma insitu may present as a broad patch or plaque that is more conducive to punch technique (Figure 9).
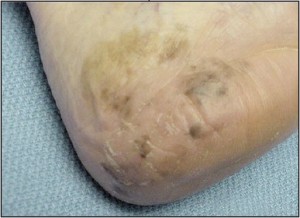 Figure 9. Melanoma in-situ may present as a large patch, which is best sampled with multiple punch biopsies (Courtesy Mark Lambert, DPM).
Figure 9. Melanoma in-situ may present as a large patch, which is best sampled with multiple punch biopsies (Courtesy Mark Lambert, DPM).
In general terms, when a small part of a patch or plaque is sampled for histopathology, punch technique is the technique of choice. One exception to the general rule that only large lesions are punched, and those that are small are shaven, involves verrucous lesions on the plantar surface. Because keratotic lesions iagof the plantar surface tend to be endophytic, shaves may not sample deep enough to obtain diagnostic tissue. For such lesions, punch technique will better allow the dermatopathologist to distinguish between lesions that may mimic each other superficially. An example of two lesions that might be identical superficially, and one that has caught me unsuspecting, are those of superficial palmoplantar wart and verrucous carcinoma.
Ulcers
Ulcers may be sampled by several methods depending largely on their clinical setting. Ulcers may be either neoplastic (carcinoma or melanoma) (Figure 10) or inflammatory (stasis or vasculitis) in etiology.
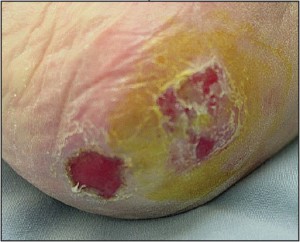 Figure 10. Neoplastic ulcers may closely resemble those that have medical etiologies.Such lesions may be easily sampled with a central and peripheral punch biopsy.
Figure 10. Neoplastic ulcers may closely resemble those that have medical etiologies.Such lesions may be easily sampled with a central and peripheral punch biopsy.
In most instances punches are ideal in either setting; however, exceptions exist. When punches are used, their ideal number and placement might vary. When characterizing suspected neoplastic ulcerations, a single punch may be all that is needed to obtain a diagnosis; however, in such conditions two are better than one to ensure appropriate sampling. In this clinical setting, one central punch and one upon the mounded flesh at the periphery of the ulcer is usually adequate. Usually relatively small punches are adequate. Alternatively, clinicians could potentially use a dermal curette or scalpel to obtain viable tissue from the ulcer’s base and proud peripheral flesh. In contrast to neoplastic ulcers, to characterize inflammatory ulcers, only punch biopsy techniques are recommended. In most cases, the cause of the ulceration in question lies deep within the dermis, far below the reaches of a superficial shave biopsy. For most conditions at least two punches are recommended, one central and one over the epidermal lip at the periphery of the ulcer. One exception to this recommendation would be for suspected vasculitis. In this context, multiple random punches are required over the ulcer base. In the case of vasculitis, the objective is to sample multiple locations along the ulcerbase in hopes of finding an affected vessel within the underlying dermis. Because this is a search that is reminiscent of depth charges seeking out an underlying submarine, there is an advantage to using punches of 3mm or larger.
Advanced superficial tumors
In most cases, the objective when biopsying advanced superficial tumors is not to rule in or out malignancy as much as it is to define the type of malignancy (Figure 11).
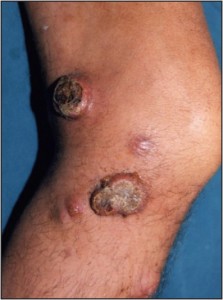 Figure 11. Advanced superficial neoplasms such as thismelanoma are sampled to precisely characterize a known malignancy.
Figure 11. Advanced superficial neoplasms such as thismelanoma are sampled to precisely characterize a known malignancy.
This allows the clinicians to know whether they should deal with the neoplasm in question themselves, or whether the patient should be referred out to an alternative subspecialist. In addition, for those cases that are to be referred out, the precise diagnosis will provide the podiatric physician with the information s/he needs to refer the patient out to the appropriate discipline. In this setting, a punch biopsy will allow the clinician diagofnostic tissue without complicating a future excision or sentinel node biopsy.
Soft tissue/subcutaneous masses
Deep soft tissue masses and those lesions which clinically resemble them were discussed briefly above. To elaborate, there are four methods of sampling masses of the soft tissue: 1) excisional biopsy, 2) incisional open biopsy, 3) core needle biopsy, 4) needle aspiration biopsy. Excisional biopsy should be reserved for bases that are small and proven to be benign.
Shaves typically extend only to the depth of the superficial dermis.
The accidental excision of an unsuspected sarcoma could have prognostic significance ranging from loss of limb to loss of life. Incisional open biopsy carries with it the advantage that a larger volume of tissue is removed, which may be useful for testing purposes. However, due to the high risk of wound site complications, and poor tumor sampling, this technique is usually initially deferred. The most common technique for sampling soft tissue masses in the extremities is core needle biopsy. This technique allows the surgeon to harvest cores of tissue from various sites within the mass in question and has a very low complication rate. The most common core needles for this indication are Tru-cut™ and Tenmo™. Needle aspiration is an excellent initial minimally invasive technique to help verify benign diagnoses (ganglia), and to help guide clinicians to more invasive biopsy techniques when indicated.
Papules or macules
Papules and macules are by definition less than 1cm in greatest dimension (Figure 12).
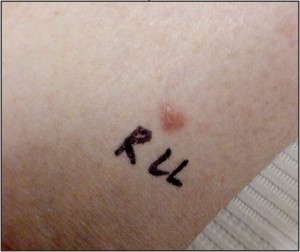 Figure 12. Papules and macules such as this basal cell carcinoma are best sampled using shave technique or saucerization (Courtesy Mark Lambert, DPM).
Figure 12. Papules and macules such as this basal cell carcinoma are best sampled using shave technique or saucerization (Courtesy Mark Lambert, DPM).
Because of their small size they can often be removed entirely, or almost entirely, using a standard shave technique or saucerization. By removing the preponderance of the lesion in question for histopathologic analysis, the dermatopathologist will be far less likely to be fooled by inadequate sampling. Papules are small elevations over the skin surface which measure less than 5mm (1cm in some texts). Because these primary lesions are elevated, they are readily shaved flush using a 15 or 10 blade. As papules become small nodules, they tend to become less superficial in extent. For relatively small nodules that seem to have a component that extends deeper into the dermis, or for macules (small flat alterations in pigment), saucerization is often a preferred biopsy technique. Because saucerization uses a metal blade that is bent to form an arch, this techniqueallows the physician to “scoop out” the biopsy site. The curve of the blade allows for better control when sampling lesions that lie deep to the skin surface.
Post-Biopsy Specimen Handling
The correct handing of the biopsy specimen, after the procedure has been performed, could make the difference between obtaining a rapid diagnosis and the performance of a wasted procedure. The overwhelming majority of tissue that is sampled using biopsy techniques should be fixed in formalin (10% formaldehyde); however, exceptions exist. The exceptions are as follows: Gout: When gout is suspected, the specimen should be placed in dehydrated alcohol. Alcohol preserves the monosodium urate crystals for crystal analysis and review under polarized light. Regardless of the biopsy method, alcohol is preferred unless the sample is kept fresh in a sterile container or a slide preparation is made in the office setting. Remember formalin contains 90% water, and water dissolves monosodiumu rate crystals.
Epidermal nerve fiber density testing: Formalin fixative is an excellent preservative for routine histopathology; however, it has deleterious effects on tissue taken for epidermal nerve fiber density analysis (degenerates intra-epidermal nerves and blocks important antibody binding sites on their surface). There are two potential fixatives that can be used for this test, each typically distributed only by labs specializing in this test (see www.bakopathology.com for more information).
Aspirations: Aspiration biopsies are ideally preserved in cytologic fixative. Formalin may dull some of the fine nuclear features that aid in diagnoses. Alternatively, slide preparations may be made in the office setting when clinicians have experience in this technique. We have made important diagnoses on aspirates that were received in formalin fixative; however, clinicians should know that this fixative has some limitations.
Cultures: Samples taken for microbiologic culture are not typically considered “biopsies” in the same sense as those procedures herein discussed and would not be billed in the same manner. Such samples are mentioned here only to stress that
tissue taken for culture should be kept fresh and not contaminated. This may be done using appropriate tissue swabs, agar slants, or sterile cups. There is a roughly 3 day window within which such specimens fixashould be plated after which bacterial cultures begin to be compromised. Formalin and alcohol will preserve tissue for histopathologic analysis, but it will kill all living cells within the sample.
The small size of most biopsies makes them particularly susceptible to air dry artifact
Most biopsies are small, either in width, depth, or both. Their small size makes them particularly susceptible to air dry artifact. Once samples that are to be analyzed histopathologically are placed in formalin fixative,they are indefinitely preserved. The case is similar with alcohol. Punch biopsies taken for epidermal nerve fiber density are unique in that they can be left in their special fixative (not formalin) for only 24 hours before the quality of the sample will begin to erode. For this reason, the specimen is typically retained within the clinical practice for 12-24 hours as it is being fixed, rinsed, and then placed in cryoprotectant for shipping.
Conclusion
In summary, there are a host of biopsy techniques within the armamentarium of today’s podiatric surgeon, each of which has its own indications. Many of the indications for these procedures are absolute and leave room for subjectivity only in unique circumstances. Once the indication has been acknowledged, the most appropriate biopsy technique should be chosen and performed. The product of most biopsies will be small ,making it necessary to quickly transfer the specimen into the correct fixasamtive. Formalin is usually the fixative of choice but exceptions do exist. Finally, although there is always an “ideal” technique for a particular indication, the fundamental principle is that “making the diagnosis trumps all else”. As physicians, we’ll virtually never do harm by doing a less than ideal procedure; however, in some instances, we will ensure harm by doing nothing at all.

Dr. Bakotic is a Fellow of the American Board of Dermatopathology, the American Board of Pathology, the College of American Pathologists , and the America Professional Wound Care Association, and is a member of the American Academy of Podiatric Practice Management. He is Director, Education and Research for Bako Pathology Services. Dr. Bakotic can be reached at brad@bakopathology.com.